Molar heat capacity
The concept is not appropriate for substances whose precise composition is not known, or whose molar mass is not well defined, such as polymers and oligomers of indeterminate molecular size.
For example, "H2O: 75.338 J⋅K−1⋅mol−1 (25 °C, 101.325 kPa)" [2] When not specified, published values of the molar heat capacity cm generally are valid for some standard conditions for temperature and pressure.
However, the dependency of cm(P,T) on starting temperature and pressure can often be ignored in practical contexts, e.g. when working in narrow ranges of those variables.
In those contexts one can usually omit the qualifier (P,T), and approximate the molar heat capacity by a constant cm suitable for those ranges.
The molar heat capacity is an "intensive" property of a substance, an intrinsic characteristic that does not depend on the size or shape of the amount in consideration.
[3]) The injection of heat energy into a substance, besides raising its temperature, usually causes an increase in its volume and/or its pressure, depending on how the sample is confined.
The temperature of a sample of a substance reflects the average kinetic energy of its constituent particles (atoms or molecules) relative to its center of mass.
And, indeed, the experimental values of cV,m for the noble gases helium, neon, argon, krypton, and xenon (at 1 atm and 25 °C) are all 12.5 J⋅K−1⋅mol−1, which is 3/2R; even though their atomic weights range from 4 to 131.
[10] For example, the molar heat capacity of nitrogen N2 at constant volume is 20.6 J⋅K−1⋅mol−1 (at 15 °C, 1 atm), which is 2.49 R.[11] From the theoretical equation cV,m = 1/2fR, one concludes that each molecule has f = 5 degrees of freedom.
Because of those two extra degrees of freedom, the molar heat capacity cV,m of N2 (20.6 J⋅K−1⋅mol−1) is greater than that of an hypothetical monatomic gas (12.5 J⋅K−1⋅mol−1) by a factor of 5/3.
According to classical mechanics, a diatomic molecule like nitrogen should have more degrees of internal freedom, corresponding to vibration of the two atoms that stretch and compress the bond between them.
That would bring f up to 7, and cV,m to 3.5 R. The reason why these vibrations are not absorbing their expected fraction of heat energy input is provided by quantum mechanics.
Therefore, if the temperature T of the system is not high enough, the average energy that would be available for some of the theoretical degrees of freedom (kT/f) may be less than the corresponding minimum quantum.
The molar heat capacity of the gas will then be determined only by the "active" degrees of freedom — that, for most molecules, can receive enough energy to overcome that quantum threshold.
[12] For each degree of freedom, there is an approximate critical temperature at which it "thaws" ("unfreezes") and becomes active, thus being able to hold heat energy.
For the three translational degrees of freedom of molecules in a gas, this critical temperature is extremely small, so they can be assumed to be always active.
The following is a table of some constant-pressure molar heat capacities cP,m of various diatomic gases at standard temperature (25 °C = 298 K), at 500 °C, and at 5000 °C, and the apparent number of degrees of freedom f* estimated by the formula f* = 2cP,m/R − 2: (*) At 59 C (boiling point) The quantum harmonic oscillator approximation implies that the spacing of energy levels of vibrational modes are inversely proportional to the square root of the reduced mass of the atoms composing the diatomic molecule.
The molar heat capacity of Br2 at room temperature is consistent with f = 7 degrees of freedom, the maximum for a diatomic molecule.
Quantum mechanics also explains why the specific heat of monatomic gases is well predicted by the ideal gas theory with the assumption that each molecule is a point mass that has only the f = 3 translational degrees of freedom.
Because the moment of inertia of a single atom is exceedingly small, the activation temperature for its rotational modes is extremely high.
The same applies to the moment of inertia of a diatomic molecule (or a linear polyatomic one) about the internuclear axis, which is why that mode of rotation is not active in general.
The following table shows the experimental molar heat capacities at constant pressure cP,m of the above polyatomic gases at standard temperature (25 °C = 298 K), at 500 °C, and at 5000 °C, and the apparent number of degrees of freedom f* estimated by the formula f* = 2cP,m/R − 2: (*) At 3000C In most solids (but not all), the molecules have a fixed mean position and orientation, and therefore the only degrees of freedom available are the vibrations of the atoms.
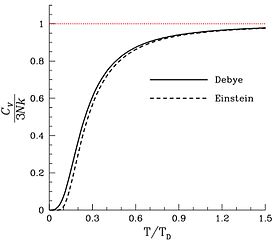
The atom-molar heat capacity of a polyatomic gas approaches that of a solid as the number n of atoms per molecule increases.
As in the case f gases, some of the vibration modes will be "frozen out" at low temperatures, especially in solids with light and tightly bound atoms, causing the atom-molar heat capacity to be less than this theoretical limit.
Water ice close to the melting point, too, has an anomalously low heat capacity per atom (1.5 R, only 50% of the theoretical value).
The specific heat has characteristic discontinuities at the glass transition temperature which are caused by the absence in the glassy state of percolating clusters made of broken bonds (configurons) that are present only in the liquid phase.
[35] Above the glass transition temperature percolating clusters formed by broken bonds enable a more floppy structure and hence a larger degree of freedom for atomic motion which results in a higher heat capacity of liquids.
Below the glass transition temperature there are no extended clusters of broken bonds and the heat capacity is smaller because the solid-state (glassy) structure of amorphous material is more rigid.
Hydrogen-containing polar molecules like ethanol, ammonia, and water have powerful, intermolecular hydrogen bonds when in their liquid phase.
These bonds provide another place where heat may be stored as potential energy of vibration, even at comparatively low temperatures.