Molecular neuroscience
In molecular biology, communication between neurons typically occurs by chemical transmission across gaps between the cells called synapses.
[3] Immunocytochemistry, which involves raising antibodies against targeted chemical or biological entities, includes a few other techniques of interest.
The presence of potassium channels was first identified in Drosophila melanogaster mutant flies that shook uncontrollably upon anesthesia due to problems in cellular repolarization that led to abnormal neuron and muscle electrophysiology.
It is notable, as with the case of neurotransmitter release, that calcium channels can interact with intracellular proteins and plays a strong role in signaling, especially in locations such as the sarcoplasmic reticulum of muscle cells.
For example, the compound diazepam (marketed as Valium) is an allosteric agonist which increases the affinity of the receptor for GABA.
On the other hand, GABA receptors can also be targeted by decreasing Cl− cellular influx with the effect of convulsants like picrotoxin.
NMDA receptors are notable for their excitatory mechanisms to affect neuronal plasticity in learning and memory, as well as neuropathologies such as stroke and epilepsy.
This Ca2+ influx is increased by excitatory postsynaptic potentials (EPSPs) produced by NMDA receptors, activating Ca2+-based signaling cascades (such as neurotransmitter release).
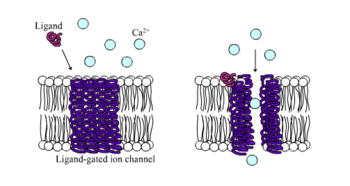
[5] Nicotinic receptors bind the acetylcholine (ACh) neurotransmitter to produce non-selective cation channel flow that generates excitatory postsynaptic responses.
Receptor activity, which can be influenced by nicotine consumption, produces feelings of euphoria, relaxation, and inevitably addiction in high levels.
The mechanism of action by which G protein-linked receptors cause a signaling cascade is as follows: Neurotransmitters are released in discrete packets known as quanta from the axon terminal of one neuron to the dendrites of another across a synapse.
Vesicular fusion with the terminal membrane and release of the neurotransmitter is caused by the generation of Ca2+ gradients induced by incoming action potentials.
The Ca2+ ions cause the mobilization of newly synthesized vesicles from a reserve pool to undergo this membrane fusion.
Recent studies seem to suggest that regulating these dimorphisms has implications for understanding normal and abnormal brain function.
[9] Many brain functions can be influenced at the cellular and molecular level by variations and changes in gene expression, without altering the sequence of DNA in an organism.
[10] Epigenetic control has been shown to be involved in high levels of plasticity in early development, thereby defining its importance in the critical period of an organism.
[11] Examples of how epigenetic changes can affect the human brain are as follows: Excitotoxicity is phenomenon in which glutamate receptors are inappropriately activated.
It is hypothesized that the deposition of amyloid-β peptide (40-42 amino acid residues) in the brain is integral in the incidence of Alzheimer's disease.
It is a hypokinetic movement basal ganglia disease caused by the loss of dopaminergic neurons in the substantia nigra of the human brain.
The inhibitory outflow of the basal ganglia is thus not decreased, and so upper motor neurons, mediated by the thalamus, are not activated in a timely manner.
This poses the opposite effects of those associated with Parkinson's disease, including inappropriate activation of upper motor neurons.
As with the GABAergic mechanisms observed in relation to Parkinson's disease, a GABA agonist injected into the substantia nigra pars reticulata decreases inhibition of upper motor neurons, resulting in ballistic involuntary motor movements, similar to symptoms of Huntington's disease.