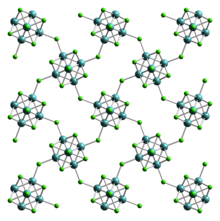
Molybdenum(II) chloride
At least two forms are known, and both have attracted much attention from academic researchers because of the unexpected structures seen for these compounds and the fact that they give rise to hundreds of derivatives.
Molybdenum(II), which is a rather large ion, prefers to form compounds with metal-metal bonds, i.e. metal clusters.
In fact all "lower halides" (i.e. where halide/M ratio is <4) in the "early transition metal series (Ti, V, Cr, Mn triads) do.
Ta and Nb form related clusters where halides are bridge edges of the Ta6 octahedron vs faces.
[3] The Mo-S clusters Mo6S8L6, analogues of the "Chevrel phases", have been prepared by the reaction of sulfide sources with Mo6Cl12 in the presence of donor ligands L.[4]