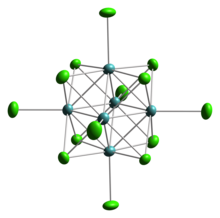
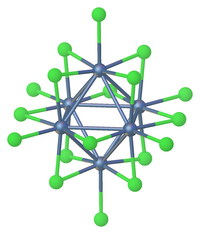
Octahedral cluster
A well-studied class of solid-state compounds related to the chalcohalides are molybdenum clusters of the type AxMo6X8 with X sulfur or selenium and Ax an interstitial atom such as Pb.
[2] Octahedral clusters of tin(II) have been observed in several solid state compounds.
[8] Crystal structures have been reported for compounds with the formula Sn6O4(OR)4, where R is an alkoxide such as a methyl or ethyl group.
[9][10] Recently, it was demonstrated that anionic tin(II) clusters [Sn6O8]4- may form the close packed arrays as in the case of α-Sn6SiO8, which adopts the zinc blende structure, comprising a face-centred-cubic array of [Sn6O8]4- clusters with Si4+ occupying half of the tetrahedral holes.
[11] A polymorph, β-Sn6SiO8, has been identified as a product of pewter corrosion in aqueous conditions, and is a structural analogue of wurtzite.
Fewer d-electrons result in weakened M-M bonding and the extended Ta---Ta distances accommodate doubly bridging halides.
A variety of analogous compounds have been reported where some or all of the Fe centres are replaced by Ru, Mn and other metals.