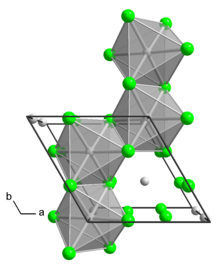
Molybdenum tetrachloride
Molybdenum tetrachloride is the inorganic compound with the empirical formula MoCl4.
The material exists as two polymorphs, both being dark-colored paramagnetic solids.
[1] In addition to these two binary phases, a number of adducts are know with the formula MoCl4L2 where L is a Lewis base.
α-Molybdenum tetrachloride can be prepared from by dechlorination of molybdenum pentachloride using tetrachloroethene:[2] Heating α-molybdenum tetrachloride in a sealed container in the presence of molybdenum pentachloride induces conversion to the β polymorph.
[2] When heated in an open container, molybdenum tetrachloride evolves chlorine, giving molybdenum trichloride;[2] The acetonitrile complex adduct can be prepared by reduction of the pentachloride with acetonitrile:[3][4] The MeCN ligands can be exchanged with other ligands: The pentachloride can be reduced to the ether complex MoCl4(Et2O)2 using tin powder.