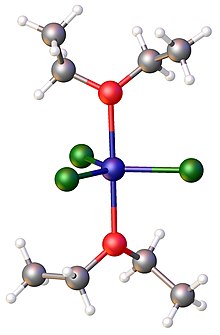
Transition metal ether complex
Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry.
[4] Being weakly basic, ether ligands tend to be easily displaceable.
Cyclic ethers such as thf can ring-open or even deoxygenated when bound to highly electrophilic metal halides.
Examples often feature weakly coordinating anions such as BArF4− and Al(ORF)4−.
[12] These compounds are often reagents because they are soluble in organic solvents as well as being anhydrous.