Multiplicity (chemistry)
In spectroscopy and quantum chemistry, the multiplicity of an energy level is defined as 2S+1, where S is the total spin angular momentum.
This spin is due to two unpaired electrons, as a result of Hund's rule which favors the single filling of degenerate orbitals.
A simple explanation of the spin states of such complexes is provided by crystal field theory.
In the ground state of dioxygen, this energy level is occupied by two electrons of the same spin, as shown in the molecular orbital diagram.
In organic chemistry, carbenes are molecules which have carbon atoms with only six electrons in their valence shells and therefore disobey the octet rule.
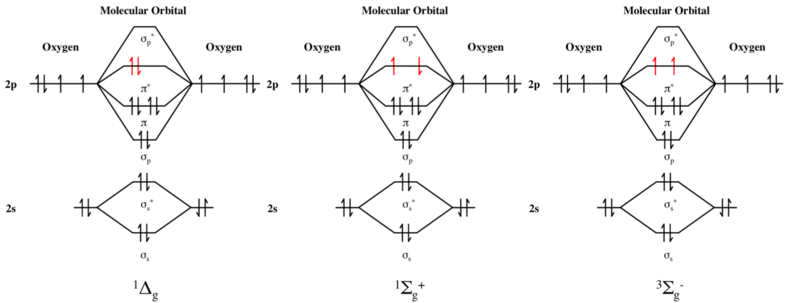
g singlet oxygen (second excited state), and 3 Σ −
g triplet oxygen (ground state).