Octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.
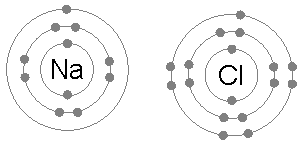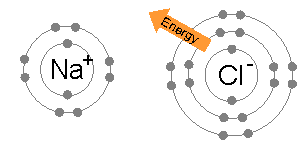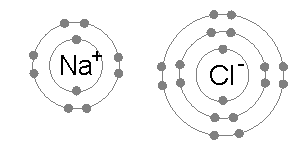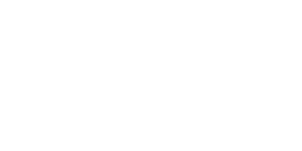
The result is that chlorine will very often form a compound in which it has eight electrons in its outer shell (a complete octet), as in Cl−.
In 1864, the English chemist John Newlands classified the sixty-two known elements into eight groups, based on their physical properties.
Abegg noted that the difference between the maximum positive and negative valences of an element under his model is frequently eight.
Walther Kossel[12] and Gilbert N. Lewis saw that noble gases did not have the tendency of taking part in chemical reactions under ordinary conditions.
On the basis of this observation, they concluded that atoms of noble gases are stable and on the basis of this conclusion they proposed a theory of valency known as "electronic theory of valency" in 1916: During the formation of a chemical bond, atoms combine together by gaining, losing or sharing electrons in such a way that they acquire nearest noble gas configuration.
According to the octet rule, the atoms immediately before and after neon in the periodic table (i.e. C, N, O, F, Na, Mg and Al), tend to attain a similar configuration by gaining, losing, or sharing electrons.
There is also an empty 3d level, but it is at considerably higher energy than 3s and 3p (unlike in the hydrogen atom), so that 3s2 3p6 is still considered a closed shell for chemical purposes.
Dioxygen is sometimes represented as obeying the octet rule with a double bond (O=O) containing two pairs of shared electrons.
[15] However the ground state of this molecule is paramagnetic, indicating the presence of unpaired electrons.
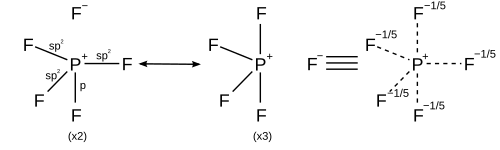
[17] To form five bonds, the one s, three p and one d orbitals combine to form five sp3d hybrid orbitals which each share an electron pair with a halogen atom, for a total of 10 shared electrons, two more than the octet rule predicts.
[18] In this model the availability of empty d orbitals is used to explain the fact that third-row atoms such as phosphorus and sulfur can form more than four covalent bonds, whereas second-row atoms such as nitrogen and oxygen are strictly limited by the octet rule.
[21][22] Nevertheless, for historical reasons, structures implying more than eight electrons around elements like P, S, Se, or I are still common in textbooks and research articles.