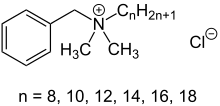
Benzalkonium chloride
Benzalkonium chloride possesses surfactant properties, dissolving the lipid phase of the tear film and increasing drug penetration,[2] making it a useful excipient, but at the risk of causing damage to the surface of the eye.
[3] Benzalkonium chloride is a mainstay of phase-transfer catalysis, an important technology in the synthesis of organic compounds, including drugs.
The FDA has stated that benzalkonium chloride is eligible as an alternative for use in the formulation of healthcare personnel hand rubs.
[13] As a hand sanitizer, use of BZK may be advantageous over ethanol in some situations because it has significantly more residual antibacterial action on the skin after initial application.
[19][20] Due to its antimicrobial activity[21] when applied to skin, some topical medications for acne vulgaris have benzalkonium chloride added to increase the products' efficiency or shelf-life.
[27] Although historically benzalkonium chloride has been ubiquitous as a preservative in ophthalmic preparations, its ocular toxicity and irritant properties,[28] in conjunction with consumer demand, have led pharmaceutical companies to increase production of preservative-free preparations, or to replace benzalkonium chloride with preservatives which are less harmful.
[32][33] In the United States, nasal steroid preparations that are free of benzalkonium chloride include budesonide, triamcinolone acetonide, dexamethasone, and Beconase and Vancenase aerosol inhalers.
[citation needed] RTECS lists the following acute toxicity data:[37] Benzalkonium chloride is a human skin and severe eye irritant.
0.1% is the maximum concentration of benzalkonium chloride that does not produce primary irritation on intact skin or act as a sensitizer.
[44] In 2018 a Japanese nurse was arrested and admitted to having murdered approximately 20 patients at a hospital in Yokohama by injecting benzalkonium chloride into their intravenous drip bags.
Enzymes, which finely control a wide range of respiratory and metabolic cellular activities, are particularly susceptible to deactivation.
Critical intermolecular interactions and tertiary structures in such highly specific biochemical systems can be readily disrupted by cationic surfactants.
[citation needed] Benzalkonium chloride solutions are fast-acting biocidal agents with a moderately long duration of action.
[49] Activity is not greatly affected by pH, but increases substantially at higher temperatures and prolonged exposure times.
[citation needed] The use of appropriate excipients can also greatly enhance the spectrum, performance and detergency, and prevent deactivation under use conditions.
[citation needed] Formulation can also help minimise deactivation of benzalkonium solutions in the presence of organic and inorganic contamination.
Benzalkonium chloride is classed as a Category III antiseptic active ingredient by the United States Food and Drug Administration (FDA).
In September 2016, the FDA announced a ban on nineteen ingredients in consumer antibacterial soaps citing a lack of evidence for safety and effectiveness.
[54] There is acknowledgement that more data are required on its safety, efficacy, and effectiveness, especially with relation to: However, recent studies have demonstrated the capacity of environmental microorganisms to develop reduced susceptibility to benzalkonium chloride by employing strategies such as modifying bacterial membranes: increasing pump activity, and reducing the expression of certain porins.