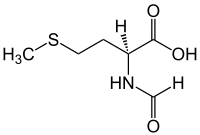
N-Formylmethionine
The 30S ribosome–mRNA complex specifically recruits tRNAs with a formylated amino acid – tRNAfMet attached to fMet in the natural case.
Given that mitochondria and chloroplasts have this initial protein synthesis with fMet in common with bacteria, this has been cited as evidence for the endosymbiotic theory.
Bacteria with their formyltransferase knocked out, which prevents Met-tRNAfMet (i.e. methionine loaded onto tRNAfMet) from turning into fMet-tRNAfMet, can have varying degrees of residual ability to start protein synthesis.
E. coli, S. pneumoniae and B. subtilis show almost no remaining translation ability, while P. aeruginosa, S. aureus, H. influenzae, and possibly S. faecalis still churn out plenty of protein.
In P. aeruginosa, this ability is facilitated by bacterial initiation factor 2, which can carry both Met-tRNAfMet and fMet-tRNAfMet to the ribosome.
Polymorphonuclear cells can bind proteins starting with fMet, and use them to initiate the attraction of circulating blood leukocytes and then stimulate microbicidal activities such as phagocytosis.
[10][11][12] Since fMet is present in proteins made by mitochondria and chloroplasts, more recent theories do not see it as a molecule that the immune system can use to distinguish self from non-self.