N-Hydroxyphthalimide
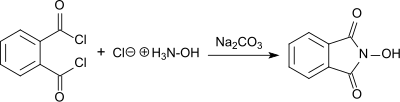
[4] The product forms as a red sodium salt under basic conditions, while white N-hydroxyphthalimide precipitates in 55% yield as the solution is acidified.
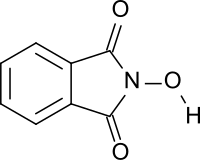
[4] Three possibilities were discussed and are shown in the Figure below: a mono-oxime of phthalic anhydride ("phthaloxime", I), an expanded ring with two heteroatoms, (2,3-benzoxazine-1,4-dione, II), and N-hydroxyphthalimide (III).
[13] Nefkens and Tesser developed a technique for generating active esters from N-hydroxyphthalimide[14] for use in peptide synthesis,[15] an approach later extended to using N-hydroxysuccinimide.
The N-hydroxyphthalimide can be removed by shaking with sodium bicarbonate,[15] but the N-hydroxysuccinimide approach shows greater reactivity and convenience, and is generally preferred.
[16][17] Esters of N-hydroxyphthalimide and activated sulfonic acids such as trifluoromethanesulfonic anhydride or p-toluenesulfonyl chloride are used as so-called photoacids, which split off protons during UV irradiation.
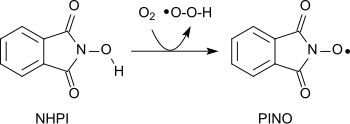
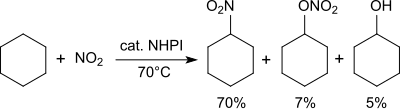
[19] A variety of functional groups can be oxidized with the aminoxyl radical (phthalimide-N-oxyl, PINO)[20] formed by the abstraction of a hydrogen atom from N-hydroxyphthalimide under gentle conditions (similar to TEMPO):[1] Using molecular oxygen alkanes can be oxidized to form alcohols, secondary alcohols to ketones, acetals to esters and alkenes to epoxides.
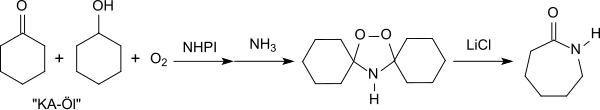
The reaction proceeds via cyclohexanol hydroperoxide, which reacts with ammonia to give peroxydicyclohexylamine followed by a rearrangement in the presence of catalytic amounts of lithium chloride.
[22][25] The use of N-hydroxyphthalimide as a catalyst in the oxidation of KA oil avoids the formation of the undesirable by-product ammonium sulfate which is produced by the conventional ε-caprolactam synthesis (Beckmann rearrangement of cyclohexanone oxime with sulfuric acid).


8 H
5 NO
3 considered as Cohn's "phthalylhydroxylamine"