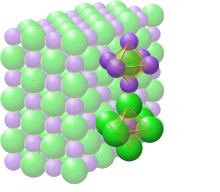
Sodium chloride
In addition to the many familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 data) include chemicals and de-icing.
Other technologies are under development due to the high energy consumption of the electrolysis, whereby small improvements in the efficiency can have large economic paybacks.
[11] It is used to flocculate and increase the density of the drilling fluid to overcome high downwell gas pressures.
Salt brine and sulfuric acid are used to coagulate an emulsified latex made from chlorinated butadiene.
The salt acts to minimize the effects of shifting caused in the subsurface by changes in humidity and traffic load.
[10] Hard water contains calcium and magnesium ions that interfere with action of soap and contribute to the buildup of a scale or film of alkaline mineral deposits in household and industrial equipment and pipes.
Commercial and residential water-softening units use ion-exchange resins to remove ions that cause the hardness.
[10][9] The second major application of salt is for deicing and anti-icing of roads, both in grit bins and spread by winter service vehicles.
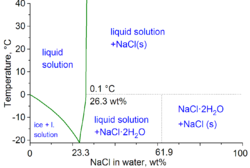
[10] In the technical terms of physical chemistry, the minimum freezing point of a water-salt mixture is −21.12 °C (−6.02 °F) for 23.31 wt% of salt.
[13] Road salt ends up in fresh-water bodies and could harm aquatic plants and animals by disrupting their osmoregulation ability.
[15] In highway de-icing, salt has been associated with corrosion of bridge decks, motor vehicles, reinforcement bar and wire, and unprotected steel structures used in road construction.
[10] A 2009 study found that approximately 70% of the road salt being applied in the Minneapolis-St Paul metro area is retained in the local watershed.
Salt is added to promote color development in bacon, ham and other processed meat products.
Salt acts as a binder in sausages to form a binding gel made up of meat, fat, and moisture.
[10] It is used as a cheap and safe desiccant because of its hygroscopic properties, making salting an effective method of food preservation historically; the salt draws water out of bacteria through osmotic pressure, keeping it from reproducing, a major source of food spoilage.
It also is used to strengthen the gluten (the elastic protein-water complex in certain doughs) and as a flavor enhancer, such as a topping on baked goods.
Sodium chloride crystals have a transmittance of at least 90% (through 1 mm) for infrared light having wavelengths in the range 0.2– 18 μm.
While inexpensive, NaCl crystals are soft and hygroscopic – when exposed to the water in ambient air, they gradually cover with "frost".
Materials that are mechanically stronger and less sensitive to moisture, such as zinc selenide and chalcogenide glasses, more widely used than NaCl.
Atomic-resolution real-time video imaging allows visualization of the initial stage of crystal nucleation of sodium chloride.
[26] The Thermal conductivity of sodium chloride as a function of temperature has a maximum of 2.03 W/(cm K) at 8 K (−265.15 °C; −445.27 °F) and decreases to 0.069 at 314 K (41 °C; 106 °F).
[30] The attraction between the Na+ and Cl− ions in the solid is so strong that only highly polar solvents like water dissolve NaCl well.
[13] The pH of a sodium chloride solution remains ≈7 due to the extremely weak basicity of the Cl− ion, which is the conjugate base of the strong acid HCl.
The existence of some of them has been experimentally confirmed at high pressures and other conditions: cubic and orthorhombic NaCl3, two-dimensional metallic tetragonal Na3Cl and exotic hexagonal NaCl.