Nanofiltration
Nanofiltration membranes have pore sizes of about 1–10 nanometers, smaller than those used in microfiltration and ultrafiltration, but a slightly bigger than those in reverse osmosis.
Membrane materials that are commonly used are polymer thin films such as polyethylene terephthalate or metals such as aluminium.
[citation needed] Historically, nanofiltration and other membrane technology used for molecular separation was applied entirely on aqueous systems.
Nanofiltration has a very favorable benefit of being able to process large volumes and continuously produce streams of products.
Repairs and replacement of membranes is dependent on total dissolved solids, flow rate and components of the feed.
[10] Concentration polarization describes the accumulation of the species being retained close to the surface of the membrane which reduces separation capabilities.
[11][12] The reason for the mesh like dimension of the spacer is to provide a hydrodynamic environment near the surface of the membrane that discourages concentration polarisation.
Flow through the tubes is normally turbulent, ensuring low concentration polarisation but also increasing energy costs.
The membranes can be easily cleaned through a 'pigging' technique with foam balls are squeezed through the tubes, scouring the caked deposits.
All of the strategies work by increasing eddies and generating a high shear in the flow near the membrane surface.
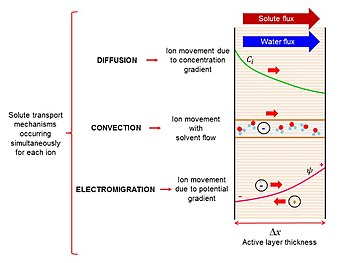
[citation needed] For charged solutes, the ionic distribution of salts near the membrane-solution interface plays an important role in determining the retention characteristic of a membrane.
[10] Unlike membranes with larger and smaller pore sizes, passage of solutes through nanofiltration is significantly more complex.
Most filtration systems operate solely by size (steric) exclusion, but at small length scales seen in nanofiltration, important effects include surface charge and hydration (solvation shell).
Solution pH strongly impacts surface charge,[15] providing a method to understand and better control rejection.
The choice and order of unit operations employed in post-treatment is dependent on water quality regulations and the design of the NF system.
[citation needed] A Polyvinyl chloride (PVC) or fibre-reinforced plastic (FRP) degasifier is used to remove dissolved gases such as carbon dioxide and hydrogen sulfide from the permeate stream.
[citation needed] The permeate water from a NF separation is demineralised and may be disposed to large changes in pH, thus providing a substantial risk of corrosion in piping and other equipment components.
To increase the stability of the water, chemical addition of alkaline solutions such as lime and caustic soda is employed.
[16] Challenges in nanofiltration (NF) technology include minimising membrane fouling and reducing energy requirements.
[18] Energy-efficient alternatives to the commonly used spiral wound arrangement are hollow fibre membranes, which require less pre-treatment.