Nanomaterials
Nanomaterials describe, in principle, chemical substances or materials of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of nanoscale[1]).
Sources of incidental nanoparticles include vehicle engine exhausts, smelting, welding fumes, combustion processes from domestic solid fuel heating and cooking.
[15] Incidental atmospheric nanoparticles are often referred to as ultrafine particles, which are unintentionally produced during an intentional operation, and could contribute to air pollution.
The structure of foraminifera (mainly chalk) and viruses (protein, capsid), the wax crystals covering a lotus or nasturtium leaf, spider and spider-mite silk,[18] the blue hue of tarantulas,[19] the "spatulae" on the bottom of gecko feet, some butterfly wing scales, natural colloids (milk, blood), horny materials (skin, claws, beaks, feathers, horns, hair), paper, cotton, nacre, corals, and even our own bone matrix are all natural organic nanomaterials.
Natural sources of nanoparticles include combustion products forest fires, volcanic ash, ocean spray, and the radioactive decay of radon gas.
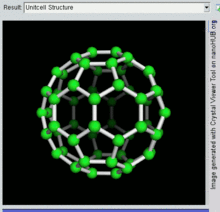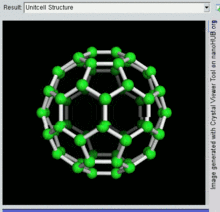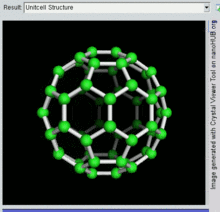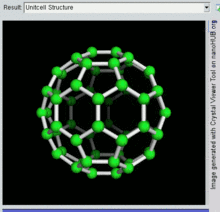
[26] The first fullerene molecule to be discovered, and the family's namesake, buckminsterfullerene (C60), was prepared in 1985 by Richard Smalley, Robert Curl, James Heath, Sean O'Brien, and Harold Kroto at Rice University.
[28] For the past decade, the chemical and physical properties of fullerenes have been a hot topic in the field of research and development, and are likely to continue to be for a long time.
In April 2003, fullerenes were under study for potential medicinal use: binding specific antibiotics to the structure of resistant bacteria and even target certain types of cancer cells such as melanoma.
Nanoparticles or nanocrystals made of metals, semiconductors, or oxides are of particular interest for their mechanical, electrical, magnetic, optical, chemical and other properties.
Recently, a range of nanoparticles are extensively investigated for biomedical applications including tissue engineering, drug delivery, biosensor.
Ferroelectric materials smaller than 10 nm can switch their polarization direction using room temperature thermal energy, thus making them useless for memory storage.
Suspensions of nanoparticles are possible because the interaction of the particle surface with the solvent is strong enough to overcome differences in density, which usually result in a material either sinking or floating in a liquid.
The often very high surface area to volume ratio of nanoparticles provides a tremendous driving force for diffusion, especially at elevated temperatures.
Nano materials are used in a variety of, manufacturing processes, products and healthcare including paints, filters, insulation and lubricant additives.
Nanomaterials can also be used in three-way-catalyst applications, which have the advantage of controlling the emission of nitrogen oxides (NOx), which are precursors to acid rain and smog.
[47] The goal of any synthetic method for nanomaterials is to yield a material that exhibits properties that are a result of their characteristic length scale being in the nanometer range (1 – 100 nm).
Examples of chaotic processes are laser ablation,[48] exploding wire, arc, flame pyrolysis, combustion,[49] and precipitation synthesis techniques.
[51] Finally, nanostructured materials with small particle size, such as zeolites and asbestos, are used as catalysts in a wide range of critical industrial chemical reactions.
[citation needed] Grain boundary refinements provide strengthening by increasing the stress required to cause intergranular or transgranular fractures.
Ramos,[58] experimental work has shown that the hardness of gold nanoparticles is much higher than their bulk counterparts, as there are stacking faults and dislocations forming that activate multiple strengthening mechanisms in the material.
[56] The resistance to pressure applied can be attributed to the line defects inside the particles as well as a dislocation that provides strengthening of the mechanical properties of the nanomaterial.
For example, the movement behavior of MoS2 [65] nanoparticles dynamic contact was directly observed in situ which led to the conclusion that fullerenes can shear via rolling or sliding.
Uncontrolled agglomeration of powders due to attractive van der Waals forces can also give rise to in microstructural inhomogeneities.
Differential stresses that develop as a result of non-uniform drying shrinkage are directly related to the rate at which the solvent can be removed, and thus highly dependent upon the distribution of porosity.
[67][68][69] In addition, any fluctuations in packing density in the compact as it is prepared for the kiln are often amplified during the sintering process, yielding inhomogeneous densification.
[73][74] The quantitative analysis of nanomaterials showed that nanoparticles, nanotubes, nanocrystalline materials, nanocomposites, and graphene have been mentioned in 400,000, 181,000, 144,000, 140,000, and 119,000 ISI-indexed articles, respectively, by September 2018.
WHO commissioned systematic reviews for all important issues to assess the current state of the science and to inform the recommendations according to the process set out in the WHO Handbook for guideline development.
[85] Handling procedures can also be improved, for example, using a nanomaterial slurry or suspension in a liquid solvent instead of a dry powder will reduce dust exposure.
[9] Engineering controls are physical changes to the workplace that isolate workers from hazards, mainly ventilation systems such as fume hoods, gloveboxes, biosafety cabinets, and vented balance enclosures.
[9] Personal protective equipment normally used for typical chemicals are also appropriate for nanomaterials, including long pants, long-sleeve shirts, and closed-toed shoes, and the use of safety gloves, goggles, and impervious laboratory coats.