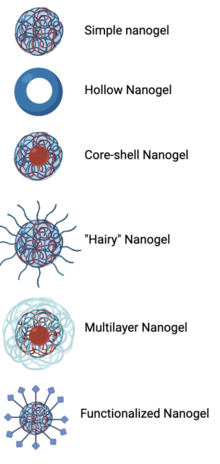
Nanogel
[1][2][3] These complex networks of polymers present a unique opportunity in the field of drug delivery at the intersection of nanoparticles and hydrogel synthesis.
[1][2][3] Nanogels can be natural, synthetic, or a combination of the two and have a high degree of tunability in terms of their size, shape, surface functionalization, and degradation mechanisms.
[1][2][3] Given these inherent characteristics in addition to their biocompatibility and capacity to encapsulate small drugs and molecules, nanogels are a promising strategy to treat disease and dysfunction by serving as delivery vehicles capable of navigating across challenging physiological barriers within the body.
[3] When thiolated polymers (thiomers) are used for this preparation process, nanogels can be further stabilized by the formation of inter- and intrachain disulfide bonds due to oxidation.
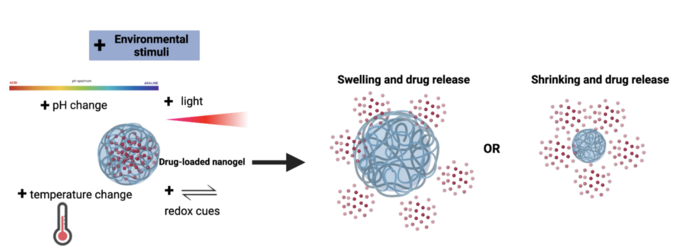
Thoughtfully designed stimuli-responsive nanogels can be leveraged to transport and release different types of cargo to specific tissues within the body with increasing spatiotemporal resolution.
[10] The protonation or deprotonation of certain functional groups can change the swelling rate and stability of a nanogel, thus resulting in the release of encapsulated cargo when exposed to different pH ranges.
[10] Temperature-responsive nanogels are a potential strategy when a therapeutic is targeting the skin, which has a natural temperature gradient, or a region experiencing inflammation.
[10][13] Given that these reducing agents and several others are found in larger concentrations inside cells compared to their external environment, redox-responsive nanogels are a promising strategy for targeted intracellular delivery.
[10] With the tunability of the wavelength of light, energy, and time of irradiation, light-responsive nanogels can be triggered to degrade with an increased control over crosslinking density.
[10] For example, both the swelling and size of light-responsive nanogels with vinyl groups were found to decrease and produce a sustained release of drugs after irradiation with UV light.
[10] One major concern with any form of drug delivery system, including nanogels, is potential side effects and damage to healthy tissue in addition to causing a negative immune response with the introduction of a foreign substance.
[3][7] The compliance and small size of degradable nanogels also allows them to travel through blood vessels and reach their target area before consumption by immune cells or filtration by the liver and spleen.
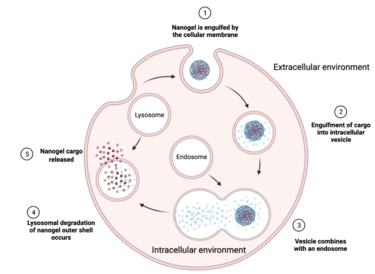
[7] At the cellular level, nanogels can be internalized by a large number of different types of endocytosis that depend on the particle’s size, shape, and surface properties.
In one study, cationic synthetic nanogels modified with insulin and transferrin were synthesized to transport oligonucleotides, a possible therapeutic and diagnostic tool for neurodegenerative disorders, to the brain.
[25] With the treatment of cardiovascular diseases in mind, polysaccharide-based nanogels have been functionalized with fucoidan to target overexpressed P-selectin receptors on platelets and endothelial cells.
In one study, nanogels loaded with nucleoside 5’-triphosphates underwent surface modifications and successfully bound to overexpressed folate receptors on breast cancer cells.
[27] Nanogels that respond to various stimuli including changes in pH and temperature or the presence of redox and light cues have proven to be useful tools for drug delivery.
[31] In addition to drug delivery applications, nanogels have been utilized as a type of imaging modality as they can encapsulate small dyes and other reporter molecules.
[7] Typical MRI contrast agents that contain gadolinium and manganese are quickly excreted from the body and carry risks of increased toxicity.
[7][39] Nanogels containing copper isotopes commonly used for PET imaging demonstrated overall stability and accumulation in tumors, which produced a higher signal in comparison to nearby tissue.
[39] Other studies have explored similar technologies with redox-responsive nanogels loaded with an isotope of gallium and other trivalent metals for PET imaging.
[41][42] For in vivo fluorescence-based optical imaging, dyes that emit NIR wavelengths >700 nm are most effective, such as indocyanine green, but encounter limitations with reduced circulation time and nonspecific interactions with other biological factors that affect the fluorescence.
[7] pH-sensitive nanogels with functionalized surface receptors to target cancer cells were loaded with a fluorescent dye that was only released upon endocytosis.
[45] Additionally, chitosan-based nanogels carrying an antibiotic, silver sulfadiazine, were found to decrease the size of second-degree burns in one in vivo study.
[47] With the goal of preventing infection and accelerating the healing process, one group has also published a new nanogel design consisting of an encapsulating core and a functionalized outer surface capable of targeting bacteria present in wounds.
[44] Boron-containing temperature-responsive nanogels formed a solid scaffold upon injection into a critical bone defect and continued to induce the production of new osteoblast cells.