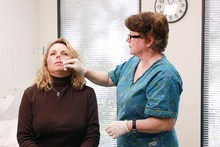
Nasal vaccine
The first live attenuated influenza vaccine (LAIV) in the form of a nasal spray was created in Russia by the Institute of Experimental Medicine in 1987.
[3] The first nasal influenza vaccine was released in the United States in 2001 but was taken off the market due to toxicity concerns.
As anthrax is an airborne substance that can be inhaled, a nasal vaccine has the potential to be used to protect individuals from the effects it can have on the respiratory system.
[5] Following the September 11, 2001 terrorist attacks in the United States, several individuals at news stations and U.S. senators died after being sent letters with anthrax as an act of bioterrorism.
[5][7] BioThrax, the current anthrax vaccine that is licensed and administered in the United States, requires up to five intramuscular injections and annual boosters; research within the past decade has developed an alternative nasal vaccine that follows the path of infection for anthrax and induces both humoral and cellular immune responses.
The mucus layer in the nasal cavity can trap smaller particles that get past the nose hairs.
[8] The nasal cavity has a large vascularization network so particles can go through the epithelial layer and directly enter the bloodstream.
The most prevalent type of nasal vaccine in research and clinical application is solutions due to its ease of use.
Nasal sprays can also be used to deliver diabetes treatment, steroids, and intranasal oxytocin to induce labor.
Nasal administration is also used to deliver anesthetics and sedatives due to direct access to the mucosal immune system and bloodstream.
Drugs and vaccines can be delivered to the brain past the blood-brain barrier through olfactory nerve cells.
[3] Nasal influenza vaccines have become popular as they reduce the risk of intramuscular injuries from administration and are painless.
[18] Virus fluid from the incubated chicken eggs is extracted and killed for the viral antigen to be purified for LAIV production.
[citation needed] In August 2020, during the COVID-19 pandemic, studies in mice and monkeys demonstrated that protection from the new coronavirus might be obtained through the nasal route.
[27] India and China approved inCOVACC and Convidecia, respectively, to be used as boosters for those who have already received at least two COVID-19 vaccine doses.
Intranasal vaccines are used on dogs for Bordetella bronchiseptica to prevent infectious tracheobronchitis (ITB).
Recent discoveries indicate that rainbow trout have a previously unknown lymphoid structure in their nasal cavity.
[34] Current research is exploring new technologies and developments to improve nasal vaccine delivery methods.
[36] Polymeric nanosystems are also being developed to deliver vaccines to target sites while preventing them from degrading; current research is focused on understanding the material and physical properties of biodegradable materials to be used in nanosystems to improve vaccine efficacy.