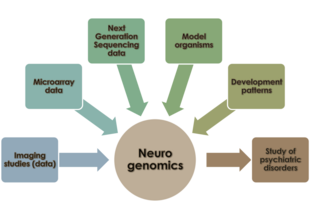
Neurogenomics
As our understanding of the genome has expanded, the role of networks of gene interactions in the maintenance of neuronal function and behaviour has garnered interest in the neuroscience research community.
Since then, global brain gene expression data, derived from microarrays, has been aligned to various behavioural quantitative trait loci (QTLs) and reported in several publications.
[15] Several independently occurring de novo mutations in patients Alzheimer's disease have been found to disrupt a shared set of functional pathways involved with neuronal signalling, for example.
[16] The quest to understand the causative biology of psychiatric disorders is hence greatly assisted by the ability to analyse entire genomes of affected and unaffected individuals in an unbiased manner.
This approach is expected to be able to identify the secondary gene networks that are disrupted in neurological disorders, subsequently assisting drug development stratagems for brain diseases.
Rare variant association studies (RVAS) have highlighted the role of de novo mutations in several congenital and early-childhood-onset disorders like autism.
[17] When autism was identified as a distinct biological disorder in the 1980s, researchers found that autistic individuals showed a brain growth abnormality in the cerebellum in their early developmental years.
[21] Subsequent research has indicated that 90% of autistic children have a larger brain volume than their peers by 2 to 4 years of age, and show an expansion in the white and gray matter content in the cerebrum.
It is hoped that an understanding of localization patterns of different psychiatric diseases could in turn inform network analysis studies in neurogenomics.
As a result, MRI scans are currently being used as a neuroimaging tool to help identify the temporal and spatial pathophysiology of Alzheimer's disease, such as specific cerebral alterations and amyloid imaging.
[16] The ease and non-invasive nature of MRI scans has motivated research projects that trace the development and onset of psychiatric diseases in the brain.
For example, MRI scans are currently being used to track the resting and task-dependent functional profiles of brains in children with autosomal dominant Alzheimer disease.
[24] Additional research has indicated that there are specifics patterns of atrophy in the cerebrum (as a repercussion of neurodegeneration) in different neurological disorders and diseases.
These disease-specific patterns of progression of atrophy can be identified with MRI scans, and provide a clinical phenotype context to neurogenomic research.
Chromosomal rearrangements, microdeletions, and large-scale translocations have been associated with impaired neurological and cognitive function, for example in hereditary neuropathy and neurofibromatosis.
Optical mapping can significantly improve variant detection and inform gene interaction network models for the diseased state in neurological disorders.
In a classic example of imaging-genomic analyses, a research study in 2012 compared MRI scans and gene expression profiles of 104 glioma patients in order to distinguish treatment outcomes and identify novel targetable genomic pathways in Glioblastoma Multiforme (GBM).
Consideration should be taken of the following factors when working with 'omics data collected from post-mortem brains: Differential diagnosis also remains a critical pre-analytical confounder of cohort-wide studies of spectrum neurological disorders.
Additionally, the RNA profiles (miRNA, ncRNA, mRNA) of the model organism need to be well catalogued, and any inferences applied from them to humans must have a basis in functional/sequence homology.
Rodent models have demonstrated behavioural phenotype changes resembling a positive schizophrenia state, either after genetic manipulation or after treatment with drugs that target the areas of the brain suspected to influence hyperactivity or neurodevelopment.
[47] It is thought that functional enrichment of genes involved in long term synaptic potentiation, depression, and plasticity has an important role to play in the acquisition, consolidation, and maintenance of traumatic memories underlying anxiety disorders.
[47] Mice models for brain research have contributed significantly to drug development and increased our understanding of the genomic underpinnings of several neurological diseases in the last generation.
Chlorpromazine, the first antipsychotic drug (discovered in 1951), was identified as a viable treatment option after it was shown to suppress response to aversive stimuli in rats in a behavioural screen.
[55] Gene expression patterns shift closer towards specialized functional profiles during embryonic development, however, certain developmental steps are still ongoing at parturition.
[54] The evolution of Homo sapiens since the divergence from the primate common ancestor has shown a marked expansion in the size and complexity of the brain, especially in the cerebral cortex.
One difficulty is that only primates have developed subdivisions in their cerebral cortex, making the modeling of human specific neurological problems difficult to mimic in rodents.
[67] Extensive research in alcohol dependence has also highlighted the need for high-quality genomic profiling of large sample sets[68][69] when studying polygenic, spectrum disorders.
[71] Studying the impact of these candidates would ideally demonstrate a schizophrenia phenotype in animal models, which is usually difficult to observe due to its manifestation as a latent personality.
[77] It is extremely difficult, however, to be able to study this in humans due to obvious restrictions with accessing the brain and retrieving biological specimens for sequencing or morphological analysis.
[75] Genomic samples of individual patients could be used to identify predictive factors, or to better understand the specific prognosis of a neurogenomic disease, and use this information to guide treatment options.