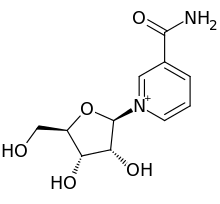
Nicotinamide riboside
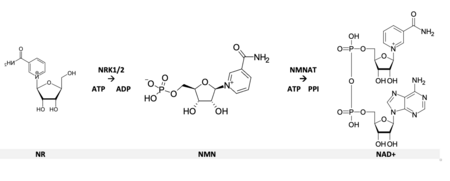
[8] V factor, purified from blood, was shown to exist in three forms: Nicotinamide adenine dinucleotide (NAD+), NMN and NR.
NAD+ (then called coenzyme I) was shown to be extremely low in cases of pellagra, and NA and NAM were identified as molecular precursors in rebuilding NAD+ levels.
NAD+ and its precursors nicotinic acid (NA) and nicotinamide (NAM) have been shown to be vital cofactors in cellular oxidation/reduction reactions and ATP synthesis.
[11][13][14] In 2004, a previously unknown pathway was reported when nicotinamide riboside (NR) was identified as an additional NAD+ precursor in eukaryotes.
NR utilization in mammals may involve both exogenous dietary sources and endogenous salvage processes that recycle intermediates.
[13][11] Disruptions or imbalances in NAD+ metabolism have been observed in many disease conditions, and the possibility of restoring NAD+ levels by administering NAD+ precursors is an area of interest for researchers.
[3][10] ChromaDex licensed patents in July 2012, and began to develop a process to bring NR to market as TruNiagen.
[19] In 2016, the U.S. Food and Drug Administration (FDA) has granted Generally recognized as safe (GRAS) status to ChromaDex for its preparation of nicotinamide riboside chloride (NRC, Niagen™).
[21] The Australian government has given nicotinamide riboside chloride a positive listing under the compositional guidelines of its Therapeutic Goods Administration (TGA).