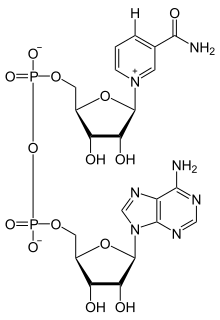
Nicotinamide adenine dinucleotide
It is also used in other cellular processes, most notably as a substrate of enzymes in adding or removing chemical groups to or from proteins, in posttranslational modifications.
[6] Such reactions (summarized in formula below) involve the removal of two hydrogen atoms from the reactant (R), in the form of a hydride ion (H−), and a proton (H+).
[15][16] In rat liver, the total amount of NAD+ and NADH is approximately 1 μmole per gram of wet weight, about 10 times the concentration of NADP+ and NADPH in the same cells.
Although most tissues synthesize NAD+ by the salvage pathway in mammals, much more de novo synthesis occurs in the liver from tryptophan, and in the kidney and macrophages from nicotinic acid.
[34] In most organisms, this enzyme uses adenosine triphosphate (ATP) as the source of the phosphate group, although several bacteria such as Mycobacterium tuberculosis and a hyperthermophilic archaeon Pyrococcus horikoshii, use inorganic polyphosphate as an alternative phosphoryl donor.
[39] Besides assembling NAD+ de novo from simple amino acid precursors, cells also salvage preformed compounds containing a pyridine base.
Some of the enzymes involved in these salvage pathways appear to be concentrated in the cell nucleus, which may compensate for the high level of reactions that consume NAD+ in this organelle.
[43] Some pathogens, such as the yeast Candida glabrata and the bacterium Haemophilus influenzae are NAD+ auxotrophs – they cannot synthesize NAD+ – but possess salvage pathways and thus are dependent on external sources of NAD+ or its precursors.
[44][45] Even more surprising is the intracellular pathogen Chlamydia trachomatis, which lacks recognizable candidates for any genes involved in the biosynthesis or salvage of both NAD+ and NADP+, and must acquire these coenzymes from its host.
In addition to these metabolic functions, NAD+ emerges as an adenine nucleotide that can be released from cells spontaneously and by regulated mechanisms,[48][49] and can therefore have important extracellular roles.
[54] An example of a NAD-binding bacterial enzyme involved in amino acid metabolism that does not have the Rossmann fold is found in Pseudomonas syringae pv.
[62] In contrast, the main function of NADPH is as a reducing agent in anabolism, with this coenzyme being involved in pathways such as fatty acid synthesis and photosynthesis.
For example, nitrifying bacteria such as Nitrobacter oxidize nitrite to nitrate, which releases sufficient energy to pump protons and generate ATP, but not enough to produce NADH directly.
[67][70] The poly(ADP-ribose) structure is involved in the regulation of several cellular events and is most important in the cell nucleus, in processes such as DNA repair and telomere maintenance.
[72] Another function of this coenzyme in cell signaling is as a precursor of cyclic ADP-ribose, which is produced from NAD+ by ADP-ribosyl cyclases, as part of a second messenger system.
[74] It does this by binding to and opening a class of calcium channels called ryanodine receptors, which are located in the membranes of organelles, such as the endoplasmic reticulum, and inducing the activation of the transcription factor NAFC3[75] NAD+ is also consumed by different NAD+-consuming enzymes, such as CD38, CD157, PARPs and the NAD-dependent deacetylases (sirtuins,such as Sir2.[76]).
[77] These enzymes act by transferring an acetyl group from their substrate protein to the ADP-ribose moiety of NAD+; this cleaves the coenzyme and releases nicotinamide and O-acetyl-ADP-ribose.
[92] Further studies are needed to determine the underlying mechanisms of its extracellular actions and their importance for human health and life processes in other organisms.
Isoniazid is a prodrug and once it has entered the bacteria, it is activated by a peroxidase enzyme, which oxidizes the compound into a free radical form.
[101] Since many oxidoreductases use NAD+ and NADH as substrates, and bind them using a highly conserved structural motif, the idea that inhibitors based on NAD+ could be specific to one enzyme is surprising.
[104] Compounds such as resveratrol increase the activity of these enzymes, which may be important in their ability to delay aging in both vertebrate,[105] and invertebrate model organisms.
Through a long and difficult purification from yeast extracts, this heat-stable factor was identified as a nucleotide sugar phosphate by Hans von Euler-Chelpin.
[113] In 1936, the German scientist Otto Heinrich Warburg showed the function of the nucleotide coenzyme in hydride transfer and identified the nicotinamide portion as the site of redox reactions.
[114] Vitamin precursors of NAD+ were first identified in 1938, when Conrad Elvehjem showed that liver has an "anti-black tongue" activity in the form of nicotinamide.
[117] In 1949, the American biochemists Morris Friedkin and Albert L. Lehninger proved that NADH linked metabolic pathways such as the citric acid cycle with the synthesis of ATP in oxidative phosphorylation.
[118] In 1958, Jack Preiss and Philip Handler discovered the intermediates and enzymes involved in the biosynthesis of NAD+;[119][120] salvage synthesis from nicotinic acid is termed the Preiss-Handler pathway.
[123] The metabolism of NAD+ remained an area of intense research into the 21st century, with interest heightened after the discovery of the NAD+-dependent protein deacetylases called sirtuins in 2000, by Shin-ichiro Imai and coworkers in the laboratory of Leonard P.
[124] In 2009 Imai proposed the "NAD World" hypothesis that key regulators of aging and longevity in mammals are sirtuin 1 and the primary NAD+ synthesizing enzyme nicotinamide phosphoribosyltransferase (NAMPT).
[125] In 2016 Imai expanded his hypothesis to "NAD World 2.0", which postulates that extracellular NAMPT from adipose tissue maintains NAD+ in the hypothalamus (the control center) in conjunction with myokines from skeletal muscle cells.
[126] In 2018, Napa Therapeutics was formed to develop drugs against a novel aging-related target based on the research in NAD metabolism conducted in the lab of Eric Verdin.