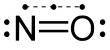Nitric oxide
[6] An important intermediate in industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms.
In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological processes.
[8] The 1998 Nobel Prize in Physiology or Medicine was awarded for discovering nitric oxide's role as a cardiovascular signalling molecule.
[9] Its impact extends beyond biology, with applications in medicine, such as the development of sildenafil (Viagra), and in industry, including semiconductor manufacturing.
[6] Nitric oxide (NO) was first identified by Joseph Priestley in the late 18th century, originally seen as merely a toxic byproduct of combustion and an environmental pollutant.
[12] Its biological significance was later uncovered in the 1980s when researchers Robert F. Furchgott, Louis J. Ignarro, and Ferid Murad discovered its critical role as a vasodilator in the cardiovascular system, a breakthrough that earned them the 1998 Nobel Prize in Physiology or Medicine.
The lone electron in the 2π orbital makes NO a doublet (X ²Π) in its ground state whose degeneracy is split in the fine structure from spin-orbit coupling with a total momentum J=3⁄2 or J=1⁄2.
The dipole of NO has been measured experimentally to 0.15740 D and is oriented from O to N (⁻NO⁺) due to the transfer of negative electronic charge from oxygen to nitrogen.
[15] Upon condensing to a neat liquid, nitric oxide dimerizes to colorless dinitrogen dioxide (O=N–N=O), but the association is weak and reversible.
The reaction is thought to proceed via the following stoichiometry: Nitric oxide reacts with fluorine, chlorine, and bromine to form the nitrosyl halides, such as nitrosyl chloride: With NO2, also a radical, NO combines to form the intensely blue dinitrogen trioxide:[6] Nitric oxide rarely sees organic chemistry use.
The Traube reaction is the addition of a two equivalents of nitric oxide onto an enolate, giving a diazeniumdiolate (also called a nitrosohydroxylamine).
[21] However, very few nucleophiles undergo the Traube reaction, either failing to adduce NO or immediately decomposing with nitrous oxide release.
In commercial settings, nitric oxide is produced by the oxidation of ammonia at 750–900 °C (normally at 850 °C) with platinum as catalyst in the Ostwald process: The uncatalyzed endothermic reaction of oxygen (O2) and nitrogen (N2), which is effected at high temperature (>2000 °C) by lightning has not been developed into a practical commercial synthesis (see Birkeland–Eyde process): In the laboratory, nitric oxide is conveniently generated by reduction of dilute nitric acid with copper: An alternative route involves the reduction of nitrous acid in the form of sodium nitrite or potassium nitrite: The iron(II) sulfate route is simple and has been used in undergraduate laboratory experiments.
The nitric oxide reacts with the ozone to produce oxygen and nitrogen dioxide, accompanied with emission of light (chemiluminescence): which can be measured with a photodetector.
Other methods of testing include electroanalysis (amperometric approach), where ·NO reacts with an electrode to induce a current or voltage change.
Nitric oxide reacts with stratospheric ozone to form O2 and nitrogen dioxide: This reaction is also utilized to measure concentrations of •NO in control volumes.
As seen in the acid deposition section, nitric oxide can transform into nitrogen dioxide (this can happen with the hydroperoxy radical, HO•2, or diatomic oxygen, O2).
[32] The binding of nitric oxide to the heme region of the enzyme leads to activation, in the presence of iron.
Sildenafil does not produce nitric oxide, but enhances the signals that are downstream of the nitric oxide pathway by protecting cyclic guanosine monophosphate (cGMP) from degradation by cGMP-specific phosphodiesterase type 5 (PDE5) in the corpus cavernosum, allowing for the signal to be enhanced, and thus vasodilation.
[30] Another endogenous gaseous transmitter, hydrogen sulfide (H2S) works with NO to induce vasodilation and angiogenesis in a cooperative manner.
The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 25 ppm (30 mg/m3) over an 8-hour workday.