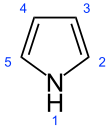
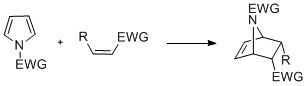Pyrrole
[5] Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use.
Substitution of pyrrole with alkyl substituents provides a more basic molecule—for example, tetramethylpyrrole has a conjugate acid pKa of +3.7.
Its name comes from the Greek pyrrhos (πυρρός, "reddish, fiery"), from the reaction used to detect it—the red color that it imparts to wood when moistened with hydrochloric acid.
Common naturally produced molecules containing pyrroles include vitamin B12, bile pigments like bilirubin and biliverdin, and the porphyrins of heme, chlorophyll, chlorins, bacteriochlorins, and porphyrinogens.
[5] Other pyrrole-containing secondary metabolites include PQQ, makaluvamine M, ryanodine, rhazinilam, lamellarin, prodigiosin, myrmicarin, and sceptrin.
[10] Pyrrole is prepared industrially by treatment of furan with ammonia in the presence of solid acid catalysts, like SiO2 and Al2O3.
[13][14] The Knorr pyrrole synthesis involves the reaction of an α-amino ketone or an α-amino-β-ketoester with an activated methylene compound.
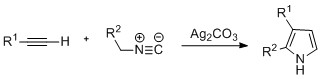
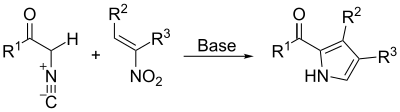
[citation needed] By the Barton–Zard synthesis, an isocyanoacetate reacts with a nitroalkene in a 1,4-addition, followed by 5-endo-dig cyclization, elimination of the nitro group, and tautomerization.
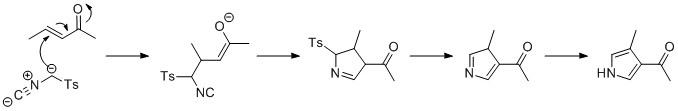
[21] The starting materials in the Piloty–Robinson pyrrole synthesis, named for Gertrude and Robert Robinson and Oskar Piloty, are two equivalents of an aldehyde and hydrazine.
Addition of hydrochloric acid leads to ring closure and loss of ammonia to form the pyrrole.
In one modification, propionaldehyde is treated first with hydrazine and then with benzoyl chloride at high temperatures and assisted by microwave irradiation:[24] Pyrroles bearing multiple substituents have been obtained from the reaction of münchnones and alkynes.
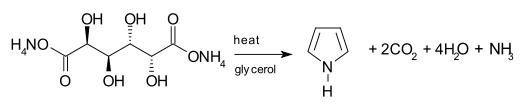
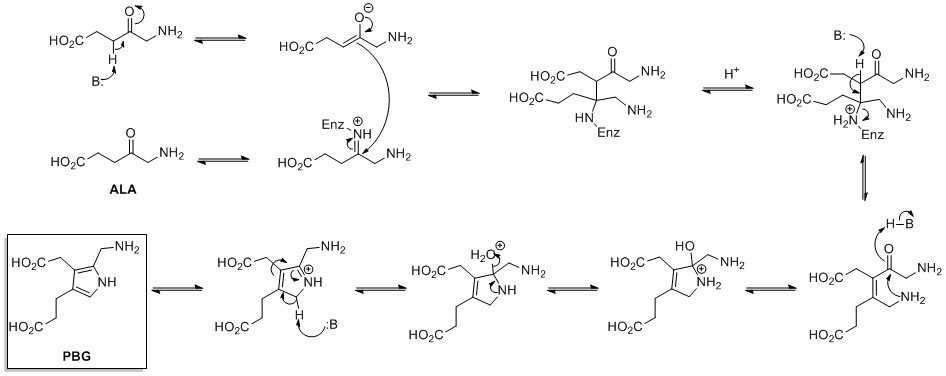
[25] The biosynthesis of pyrrole rings begins with aminolevulinic acid (ALA), which is synthesized from glycine and succinyl-CoA.
The biosynthesis of Prodigiosin[28][29] involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from L-proline, L-serine, L-methionine, pyruvate, and 2-octenal.
This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of L-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring.
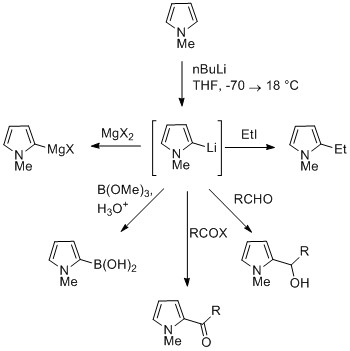
[11] Pyrroles generally react with electrophiles at the α position (C2 or C5), due to the highest degree of stability of the protonated intermediate.
Pyrroles react easily with nitrating (e.g. HNO3/Ac2O), sulfonating (Py·SO3), and halogenating (e.g. NCS, NBS, Br2, SO2Cl2, and KI/H2O2) agents.
Nitrophilic metals, such as MgX, lead to alkylation at C (mainly C2), due to a higher degree of coordination to the nitrogen atom.
[citation needed] Substitution at C3 can be achieved through the use of N-substituted 3-bromopyrrole, which can be synthesized by bromination of N-silylpyrrole with NBS.
[36] For example, Birch reduction of pyrrole esters and amides produced pyrrolines, with the regioselectivity depending on the position of the electron-withdrawing group.
Diels-Alder cyclizations can occur with the pyrrole acting as a diene, especially in the presence of an electron-withdrawing group on the nitrogen.









![Piloty–Robinson reaction[24]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/bd/Piloty-Robinson_reaction.png/400px-Piloty-Robinson_reaction.png)