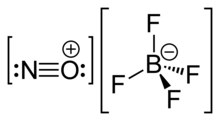Nitrosonium tetrafluoroborate
[2] The dominant property of NOBF4 is the oxidizing power and electrophilic character of the nitrosonium cation.
It forms colored charge transfer complexes with hexamethylbenzene and with 18-crown-6.
The latter, a deep yellow species, provides a means to dissolve NOBF4 in dichloromethane.
[3] Nitrosonium tetrafluoroborate may be used to prepare metal salts of the type [MII(CH3CN)x][BF4]2 (M = Cr, Mn, Fe, Co, Ni, Cu).
[5] In its infrared spectrum of this salt, νNO is a strong peak at 2387 cm−1.