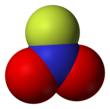
Nitryl fluoride
The structure features planar nitrogen with a short N-F bond length of 135 pm.
[2] Henri Moissan and Paul Lebeau recorded the preparation of nitryl fluoride in 1905 by the fluorination of nitrogen dioxide.
The simplest method avoids fluorine gas but uses cobalt(III) fluoride:[3] The CoF2 can be regenerated to CoF3.
The standard heat of formation of FNO2 is -19 ± 2 kcal/mol, but the compound becomes increasingly unstable at higher temperature.
[5] Nitryl fluoride can be used to prepare organic nitro compounds and nitrate esters.