Nodularin
[2] This aquatic, photosynthetic cyanobacterium forms visible colonies that present as algal blooms in brackish water bodies throughout the world.
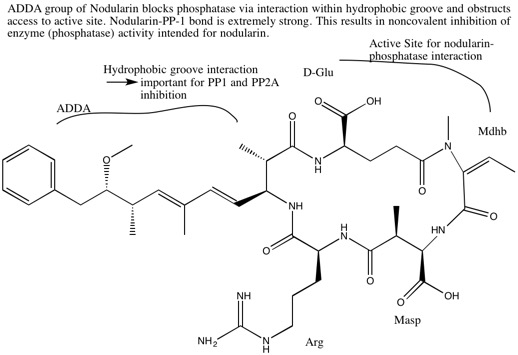
[18] A further interaction involves a Michael-addition covalent linkage of electrophilic α, β unsaturated carbonyl of a methyldehydroalanine residue on the nodularin to a thiol of cysteine 273 on PP-1.
More specifically, this redistribution leads to collapse of actin microfilaments in the hepatocyte cytoskeleton and dislocation of a-actinin and talin.
Contact with neighboring cells is reduced and sinusoidal capillaries lose stability which rapidly leads to intrahepatic hemorrhage and often results in serious liver malfunction or death.
Their tumor-promoting activity is much stronger than that of microcystins; this is believed to be due to the smaller ring structure of nodularins, which enables them to be more easily taken into hepatocytes.
This tumor promoting activity is achieved through induced gene expression of TNF-alpha and proto-oncogenes, though the exact mechanism is unknown.
Considered from a public health and epidemiologic standpoint, there is a correlation of primary liver cancer in areas of China with nodularins and microcystins in the water of ponds, ditches, rivers, and shallow wells.
[23] Experiments in rats, where animals were exposed to non-lethal doses of nodularin, provided evidence of its carcinogenicity via tumor-initiating and tumor promoting activity.
[24] Symptoms of exposure include blistering around the mouth, sore throat, headache, abdominal pain, nausea and vomiting, diarrhea, dry cough and pneumonia.
These symptoms include jaundice, bleeding easily, swollen abdomen, mental disorientation or confusion, sleepiness or coma.
[29] Blowing wind can spread substances from cyanobacterial blooms up to 10 km, increasing the area of potential exposure.
The provisional safety guideline of nodularins is 1 microgram/ L. Lethal dose (LD) oral toxicity is estimated from microcystins and reported as 5 mg/kg.
[32] Research has indicated that treating during and after with melatonin (dose: 15 mg/kg of body weight) may have protective functions against oxidative stress and damage induced by nodularins.
[33] At risk populations for nodularin poisoning are human individuals, animals, and plants living within 10 km radius of seashore and lakefront areas.
Safety guidelines can be implemented to reducing risk, specifically involving the cleanliness standards of drinking water.
Microorganisms have been proven effective in the biodegradation and removal of nodularins, which could be useful in controlling cyanobacterial blooms in public water supplies.
Protective clothing and physically avoiding areas of visible cyanobacterial blooms help reduce accidental exposures.
Synthesis is conducted by multienzyme complexes, including peptide synthetases, polypeptide synthases, and tailoring enzymes.
It was debated which class of compounds were the original hepatotoxin: recent authors argue for nodularin having evolved from the microcystin synthesis machinery,[2][35] while some older articles support the opposite.