Nonmetallic material
In everyday life it would be a generic term for those materials such as plastics, wood or ceramics which are not typical metals such as the iron alloys used in bridges.
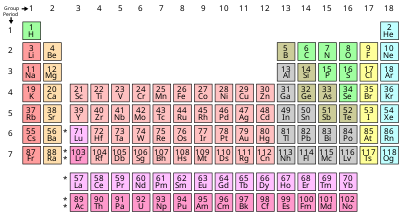
In some areas of chemistry, particularly the periodic table, it is used for just those chemical elements which are not metallic at standard temperature and pressure conditions.
[4][5] In early work[6][7] this band structure interpretation was based upon a single-electron approach with the Fermi level in the band gap as illustrated in the Figure, not including a complete picture of the many-body problem where both exchange and correlation terms can matter, as well as relativistic effects such as spin-orbit coupling.
[8] For instance, nickel oxide would be a metal if a single-electron approach was used, but in fact has quite a large band gap.
As discussed by both the chemist Peter Edwards and colleagues,[15] as well as Fumiko Yonezawa,[2]: 257–261 it is also important in practice to consider the temperatures at which both metals and nonmetals are used.
[18]) There are many experimental methods of checking for nonmetals by measuring the band gap, or by ab-initio quantum mechanical calculations.
For instance, in this usage plastics are nonmetals, but in fact there are (electrically) conducting polymers[22][23] which should formally be described as metals.
[24] A general introduction to much of this can be found in the 2017 book by Fumiko Yonezawa[2]: Chpt 1 The term nonmetal (chemistry) is also used for those elements which are not metallic in their normal ground state; compounds are sometimes excluded from consideration.
The term is sometimes also used when describing dopants of specific elements types in compounds, alloys or combinations of materials, using the periodic table classification.
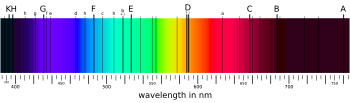
In 1802, William Hyde Wollaston[31] noted the appearance of a number of dark features in the solar spectrum.
One example is a field-effect transistor where an electric field can lead to a region where there are no electrons at the Fermi energy (depletion zone).