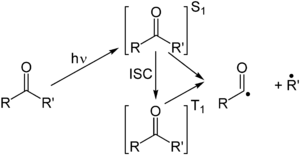
Norrish reaction
The Norrish Type I reaction is particularly significant here because it involves the cleavage of a carbon-carbon bond in a photoinitiator molecule upon excitation by UV or visible light, leading to the formation of two radical species.
This makes the Norrish Type I reaction a fundamental mechanism for designing photoinitiators that are capable of driving high-resolution additive manufacturing at the microscale.
[11] The Norrish reaction has been studied in relation to environmental chemistry with respect to the photolysis of the aldehyde heptanal, a prominent compound in Earth's atmosphere.
An example of a synthetically useful Norrish type II reaction can be found early in the total synthesis of the biologically active cardenolide ouabagenin by Phil Baran and coworkers.
[15] The optimized conditions minimize side reactions, such as the competing Norrish type I pathway, and furnish the desired intermediate in good yield on a multi-gram scale.