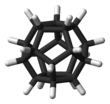
Dodecahedrane
Dodecahedrane is a chemical compound, a hydrocarbon with formula C20H20, whose carbon atoms are arranged as the vertices (corners) of a regular dodecahedron.
[4] The molecule has perfect icosahedral (Ih) symmetry, as evidenced by its proton NMR spectrum in which all hydrogen atoms appear at a single chemical shift of 3.38 ppm.
Leo Paquette's group at Ohio State University was the first to succeed, by a complex 29-step route that mostly builds the dodecahedral skeleton one ring at a time, and finally closes the last hole.
Following that idea, joint efforts of the Prinzbach team and the Schleyer group succeeded but obtained only 8% yield for the conversion at best.
In the first leg of the procedure [10] two molecules of cyclopentadiene 1 are coupled together by reaction with elemental sodium (forming the cyclopentadienyl complex) and iodine to dihydrofulvalene 2.
The final 6 carbon atoms are inserted in a nucleophilic addition to the ketone groups of the carbanion 10 generated from allyltrimethylsilane 9 and n-butyllithium.
The double bond is reduced with hydrazine and sequential diisobutylaluminum hydride reduction and pyridinium chlorochromate oxidation of 21 forms the aldehyde 22.
A second Norrish reaction then adds another C–C bond to alcohol 23 and having served its purpose the phenoxy tail is removed in several steps: a Birch reduction to diol 24, oxidation with pyridinium chlorochromate to ketoaldehyde 25 and a reverse Claisen condensation to ketone 26.
A third Norrish reaction produces alcohol 27 and a second dehydration 28 and another reduction 29 at which point the synthesis is left completely without functional groups.
The missing C-C bond is put in place by hydrogen pressurized dehydrogenation with palladium on carbon at 250 °C to dodecahedrane 30.
Half or more of the hydrogen atoms can be substituted by hydroxyl groups to yield polyols, but the extreme compound C20(OH)20 remained elusive as of 2006.
Cross, Saunders and Prinzbach succeeded in encapsulating helium atoms in dodecahedrane by shooting He+ ions at a film of the compound.