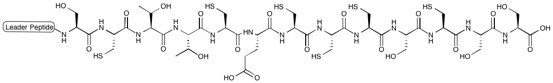
Nosiheptide
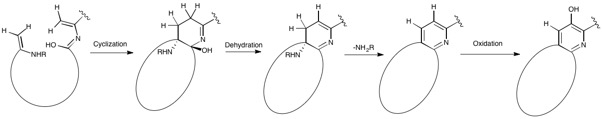
[1][2][3] Nosiheptide is classified, along with several others, as an e series thiopeptide characterized by a nitrogen containing, 6-membered heterocycle in a 2,3,5,6 substituted fashion central to multiple azoles (or azolines) and dehydroamino acids along with a macrocyclic core.
[1][2][4][5] Thiopeptides such as nosiheptide have potent activity against various bacterial pathogens, primarily gram positive, including methicillin-resistant Staphylococcus aureus, penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant enterococci.
[2][6] Nosiheptide consists of 5 thiazole rings, a central tetrasubstituted pyridine moiety, and a bicyclic macrocycle, which includes a modified amino acid (from tryptophan) external to the initial peptide translated from the gene encoding it.
[3][5] Nosiheptide and other thiopeptide's mechanism of action stems from the tight binding on the 50S ribosomal subunit and inhibiting the activities of elongation factors, preventing protein synthesis.
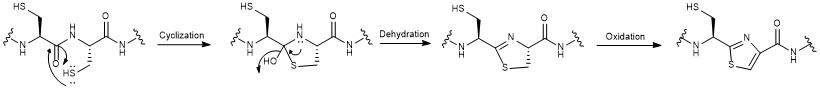
[2][4][7] Next, cyclizations occur between 5 of the cysteine residues in the structural sequence and the previous carbonyl on the adjacent amino acid to form thiazole rings after oxidation.