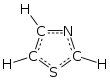
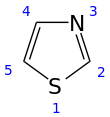
Thiazole
Being planar thiazoles are characterized by significant pi-electron delocalization and have some degree of aromaticity, more so than the corresponding oxazoles.
The calculated pi-electron density marks C5 as the primary site for electrophilic substitution, and C2-H as susceptible to deprotonation.
Thiazoles are found in a variety of specialized products, often fused with benzene derivatives, the so-called benzothiazoles.
[4] In the Cook-Heilbron synthesis, thiazoles arise by the condensation of α-aminonitrile with carbon disulfide.
Thiazoles are generally formed via reactions of cysteine, which provides the N-C-C-S backbone of the ring.
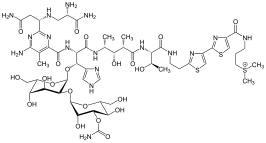
Several biosynthesis routes lead to the thiazole ring as required for the formation of thiamine.
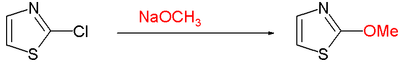
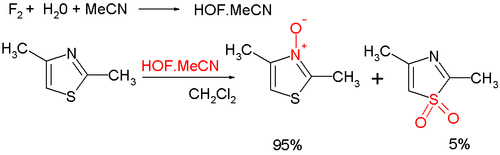
[7] Electrophilic aromatic substitution at C5 but require activating groups such as a methyl group, as illustrated in bromination: Oxidation at nitrogen gives the aromatic thiazole N-oxide; many oxidizing agents exist, such as mCPBA; a novel one is hypofluorous acid prepared from fluorine and water in acetonitrile; some of the oxidation takes place at sulfur, leading to non-aromatic sulfoxide/sulfone:[8] Thiazole N-oxides are useful in Palladium-catalysed C-H arylations, where the N-oxide is able to shift the reactivity to reliably favor the 2-position, and allows for these reactions to be carried out under much more mild conditions.