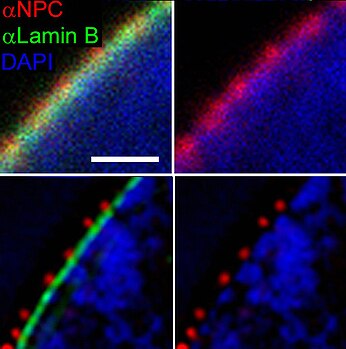
Nuclear pore complex
[2] In 2022 around 90% of the structure of the human NPC was elucidated in an open and a closed conformation, and published in a special issue of Science, featured on the cover.
Conversely, the remaining nucleoporins exhibit characteristics of "natively unfolded" or intrinsically disordered proteins, characterized by high flexibility and a lack of ordered tertiary structure.
The central region of the pore may exhibit a plug-like structure; however, its precise nature remains unknown, and it is yet undetermined whether it represents an actual plug or merely cargo transiently caught in transit.
The mammalian NPC has a molecular mass of about 124 megadaltons (MDa), comprising approximately 30 distinct protein components, each in multiple copies.
[17] The nuclear pore complex (NPC) serves as a highly regulated gateway for the transport of molecules between the nucleus and the cytoplasm.
These are a superfamily of nuclear transport receptors that facilitate the translocation of proteins, RNAs, and ribonuclear particles across the NPC in a Ran GTP hydrolase-dependent process.
Three models have been suggested to explain the translocation mechanism: Nuclear proteins are synthesized in the cytoplasm and need to be imported through the NPCs into the nucleus.
[citation needed] Importation begins with Importin-α binding to the NLS sequence of cargo proteins, forming a complex.
Then the cellular apoptosis susceptibility protein (CAS), an exportin which in the nucleus is bound to RanGTP, displaces Importin-α from the cargo.
The Importinβ-RanGTP and Importinα-CAS-RanGTP complex diffuses back to the cytoplasm where GTPs are hydrolyzed to GDP leading to the release of Importinβ and Importinα which become available for a new NLS-protein import round.
This gradient arises from the exclusive nuclear localization of RanGEFs, proteins that exchange GDP to GTP on Ran molecules.
[citation needed] In addition to nuclear import, certain molecules and macromolecular complexes, such as ribosome subunits and messenger RNAs, require export from the nucleus to the cytoplasm.
The resulting CRM1-RanGDP complex returns to the nucleus, where RanGEFs catalyze the exchange of GDP for GTP on Ran, replenishing the system's energy source.
[21] Since the NPC regulates genome access, its presence in significant quantities during cell cycle stages characterized by high transcription rates is crucial.
This disassembly of the NPC peripheral groups is largely thought to be phosphate driven, as several of these nucleoporins are phosphorylated during the stages of mitosis.
This change may make the NPC more permeable to enzymes involved in the degradation of the NE such as cytoplasmic tubulin, as well as allowing the entry of key mitotic regulator proteins.
In organisms that undergo a semi-open mitosis such as the filamentous fungus Aspergillus nidulans, 14 out of the 30 nucleoporins disassemble from the core scaffold structure, driven by the activation of the NIMA and Cdk1 kinases that phosphorylate nucleoporins and open nuclear pores[26][27] thereby widening the nuclear pore and allowing the entry of mitotic regulators.
[28] In fungi undergoing closed mitosis, where the nucleus remains intact, changes in the permeability barrier of the nuclear envelope are attributed to alterations within the NPC.