Nylon
Nylon polymers have extensive commercial applications, including uses in textiles and fibers (such as apparel, flooring and rubber reinforcement), molded components for automotive and electrical equipment, and films (mostly for food packaging).
[8]: 8, 64, 236 DuPont's invention of nylon spanned an eleven-year period, ranging from the initial research program in polymers in 1927 to its announcement in 1938, shortly before the opening of the 1939 New York World's Fair.
[13][14] In response to Carothers' work, Paul Schlack at IG Farben developed nylon 6, a different molecule based on caprolactam, on January 29, 1938.
The reactants of nylon soon constituted half of the Ammonia Department's sales and helped them come out of the period of the Great Depression by creating jobs and revenue at DuPont.
[8] The ability to acquire a large number of chemists and engineers quickly was a huge contribution to the success of DuPont's nylon project.
Nylon's commercial announcement occurred on October 27, 1938, at the final session of the Herald Tribune's yearly "Forum on Current Problems", on the site of the approaching New York City world's fair.
[10][11]: 141 The "first man-made organic textile fiber" which was derived from "coal, water and air" and promised to be "as strong as steel, as fine as the spider's web" was received enthusiastically by the audience, many of them middle-class women, and made the headlines of most newspapers.
Nylon was even mentioned by President Roosevelt's cabinet, which addressed its "vast and interesting economic possibilities" five days after the material was formally announced.
[11] Also, DuPont executives marketing nylon as a revolutionary man-made material did not at first realize that some consumers experienced a sense of unease and distrust, even fear, towards synthetic fabrics.
[11]: 126–128 A particularly damaging news story, drawing on DuPont's 1938 patent for the new polymer, suggested that one method of producing nylon might be to use cadaverine (pentamethylenediamine),[a] a chemical extracted from corpses.
During their first year on the market, an astounding 64 million pairs of nylon stockings were sold, reflecting the fabric's rapid integration into daily life and fashion.
[8]: 101 Such was the success of nylon that in 1941, just a year after its launch, a second plant was opened in Martinsville, Virginia, to meet the growing demand and ensure a steady supply of this popular fabric.
This expansion underscored the profound impact nylon had on the textile industry and its rapid rise to prominence as a versatile and sought-after material.
[22] In the meantime, women cut up nylon tents and parachutes left from the war in order to make blouses and wedding dresses.
[42] In Britain, in November 1951, the inaugural address of the 198th session of the Royal Society for the Encouragement of Arts, Manufactures and Commerce focused on the blending of textiles.
2, 1978) explained that the name was originally intended to be "No-Run" ("run" meaning "unravel") but was modified to avoid making such an unjustified claim.
[20][54][55] In the case of nylons that involve reaction of a diamine and a dicarboxylic acid, it is difficult to get the proportions exactly correct, and deviations can lead to chain termination at molecular weights less than a desirable 10,000 daltons.
To overcome this problem, a crystalline, solid "nylon salt" can be formed at room temperature, using an exact 1:1 ratio of the acid and the base to neutralize each other.
The synthetic route using lactams (cyclic amides) was developed by Paul Schlack at IG Farben, leading to nylon 6, or polycaprolactam—formed by a ring-opening polymerization.
[77] Because of the expense and difficulties of the nylon recycling process, few companies utilize it while most favor using cheaper, newly made plastics for their products instead.
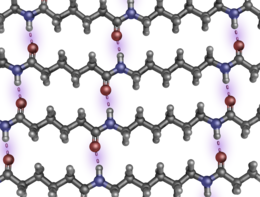
[82][83] Above their melting temperatures, Tm, thermoplastics like nylon are amorphous solids or viscous fluids in which the chains approximate random coils.
Thus parallel (but not antiparallel) strands can participate in extended, unbroken, multi-chain β-pleated sheets, a strong and tough supermolecular structure similar to that found in natural silk fibroin and the β-keratins in feathers.
If subjected to cold drawing afterwards, the fibers align further, increasing their crystallinity, and the material acquires additional tensile strength.
Bill Pittendreigh, DuPont, and other individuals and corporations worked diligently during the first few months of World War II to find a way to replace Asian silk and hemp with nylon in parachutes.
[91][6]: 514 Molded nylon is used in hair combs and mechanical parts such as machine screws, gears, gaskets, and other low- to medium-stress components previously cast in metal.
[96] Such thermoplastic composites (25% to 30% glass fiber) are frequently used in car components next to the engine, such as intake manifolds, where the good heat resistance of such materials makes them feasible competitors to metals.
Its various properties also make it very useful as a material in additive manufacturing; specifically, as a filament in consumer and professional grade fused deposition modeling 3D printers.
[103] In 1946, Segovia and string maker Albert Augustine were introduced by their mutual friend Vladimir Bobri, editor of Guitar Review.
After three years of development, Augustine demonstrated a nylon first string whose quality impressed guitarists, including Segovia, in addition to DuPont.
Eventually, however, after experimenting with various types of metal and smoothing and polishing techniques, Augustine was also able to produce high quality nylon wound strings.