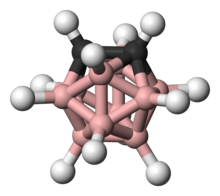
ortho-Carborane
The organic substituents are removed by ester hydrolysis followed by oxidation:[2] Upon heating to 420 °C, it rearranges to form the meta isomer.
Dicarbollides, being strong electron donors, stabilize higher oxidation states, e.g. Ni(IV).
The CH vertices of closo-dicarbadodecaboranes undergo deprotonation upon treatment with organolithium reagents:[9] These dilithiated compounds react with a variety of electrophiles, e.g. chlorophosphines, chlorosilanes, and sulfur.
[10] Many of the same compounds can be produced by hydroboration of alkynes: ortho-Carborane can be converted to highly reactive carborynes with the formula B10C2H10.
[14] Iodinated derivatives of carborane can be further modified to access boron alkylated products via a cross coupling reaction.
This can be done by treating the halogenated carborane with a Grignard reagent in the presence of a phosphine palladium complex.
The reaction mixture is allowed to stir at room temperature for two days, forming a copper-metalated carborane cage.
The crude product is then purified via column chromatography and affords one half-equivalent of the carbon-carbon linked dimer of the original ortho-carborane in high yields.