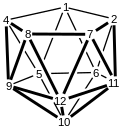
Dicarbollide
The conversion is conducted in two-steps, first "deboronation" and second deprotonation:[2] The dianion derived from dicarboranes, [C2B9H11]2-, are nido clusters.
[3] A variety of complexes - a subset of metallaborane - are known with one or two dicarbollide ligands.
Thus, these are prepared by salt metathesis reactions, as illustrated by the synthesis of the ferrocene analogue: These bisdicarbollide dianions are often readily oxidized.
In some cases, the oxidation induces rearrangement of the C2B9 cage to give complexes where the carbon centers are nonadjacent.
[6] The clam-shell dicarbollide complex (Cp*)(C2B9H11)ZrCH3 catalyzes alkene polymerization.