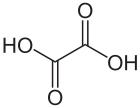
Oxalic acid
Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis, commonly known as wood-sorrels.
It is used as a cleaning agent, especially for the removal of rust, because it forms a water-soluble ferric iron complex, the ferrioxalate ion.
[10] By 1773, François Pierre Savary of Fribourg, Switzerland had isolated oxalic acid from its salt in sorrel.
[14] In 1824, the German chemist Friedrich Wöhler obtained oxalic acid by reacting cyanogen with ammonia in aqueous solution.
[34] Evaporation of a solution of urea and oxalic acid in 2:1 molar ratio yields a solid crystalline compound H2C2O4·2CO(NH2)2, consisting of stacked two-dimensional networks of the neutral molecules held together by hydrogen bonds with the oxygen atoms.
Members of the spinach family and the brassicas (cabbage, broccoli, brussels sprouts) are high in oxalates, as are sorrel and umbellifers like parsley.
[37] The leaves and stems of all species of the genus Chenopodium and related genera of the family Amaranthaceae, which includes quinoa, contain high levels of oxalic acid.
[16] Plants of the genus Fenestraria produce optical fibers made from crystalline oxalic acid to transmit light to subterranean photosynthetic sites.
[45] Cryphonectria parasitica may excrete oxalic acid containing solutions at the advancing edge of its chestnut cambium infection.
[48] LDH catalyses the conversion of pyruvate to lactic acid (end product of the fermentation (anaerobic) process) oxidising the coenzyme NADH to NAD+ and H+ concurrently.
Plants normally produce it in small amounts, but some pathogenic fungi such as Sclerotinia sclerotiorum cause a toxic accumulation.
Oxalobacter formigenes is an important gut bacterium that helps animals (including humans) degrade oxalate.
[51] Oxalic acid's main applications include cleaning or bleaching, especially for the removal of rust (iron complexing agent).
Its utility in rust removal agents is due to its forming a stable, water-soluble salt with ferric iron, ferrioxalate ion.
It is also used in bleaches, especially for pulpwood, cork, straw, cane, feathers, and for rust removal and other cleaning, in baking powder, and as a third reagent in silica analysis instruments.
[52] Dilute solutions (0.05–0.15 M) of oxalic acid can be used to remove iron from clays such as kaolinite to produce light-colored ceramics.
Oxalic acid is also widely used as a wood bleach, most often in its crystalline form to be mixed with water to its proper dilution for use.
[68] Ingestion of ethylene glycol results in oxalic acid as a metabolite which can also cause acute kidney failure.