Oxoammonium-catalyzed oxidation
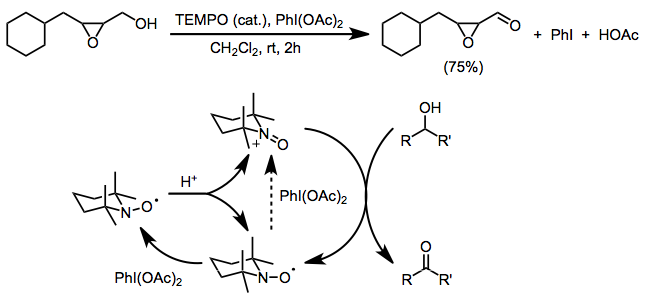
[2] The nitroxide can be used as a catalyst in conjunction with cheaper stoichiometric oxidants such as sodium hypochlorite[3] or bis(acetoxy)iodobenzene (BAIB).
[4] Under neutral or slightly acidic conditions (in the presence of silica gel, for instance), oxidation occurs by an initial hydrogen bond between the hydroxyl group and the oxoammonium nitrogen, followed by concerted proton transfer and hydride abstraction.
Attack of the substrate alkoxide on either nitrogen or oxygen may occur, although the former is believed to operate on the basis of on observations of oxidations of N-alkoxy amines (which, presumably, proceed via intermediate 1).
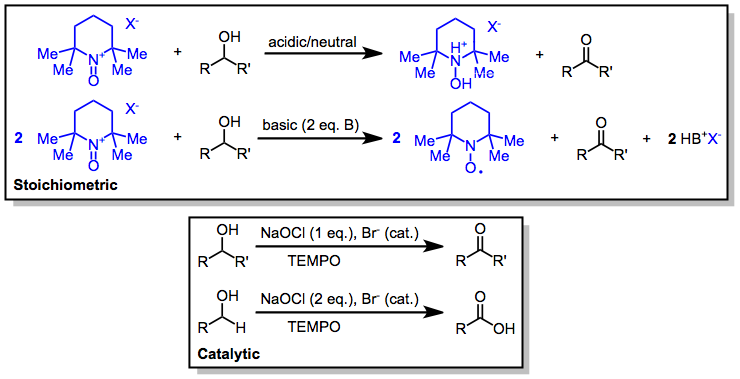
[10] (6)Oxidations using oxoammonium salts may be carried out either in the stoichiometric or catalytic mode under acidic or basic conditions.
This section describes the most commonly used conditions for the stoichiometric and catalytic oxidation of alcohols to carbonyl compounds with oxoammonium salts.
(9)Tertiary allylic alcohols can also be stoichiometrically oxidized by oxoammonium salts to enones in a variation of the Babler-Dauben reaction.
(10)The use of chlorites as terminal oxidants in conjunction with both hypochlorites and TEMPO gives carboxylic acids without chlorination side products.
[16] (11)A significant limitation of both of the above methods is incompatibility with free amine or alkene functionality, both of which undergo competitive oxidation.
BAIB is unable to oxidize the nitroxide radical directly, and initial formation of oxoammonium is believed to be due to acid-catalyzed disproportionation.
(13)Activated manganese dioxide, which oxidizes allylic and benzylic alcohols, is cheaper than TEMPO and operationally simple to use.
[22] Chromium-based reagents such as pyridinium chlorochromate can also be used to convert alcohols to carbonyl compounds; although the stoichiometric generation of chromium wastes is a disadvantage.
Dess-Martin periodinane is a highly selective, mild oxidant of alcohols, whose primary disadvantages are difficulties with preparation and safety.