Pyridinium chlorochromate
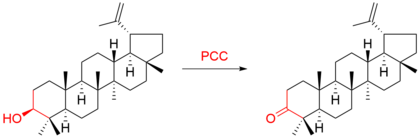
It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls.
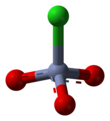
[1] PCC consists of a pyridinium cation, [C5H5NH]+, and a tetrahedral chlorochromate anion, [CrO3Cl]−.
Discovered by accident,[3] the reagent was originally prepared via addition of pyridine into a cold solution of chromium trioxide in concentrated hydrochloric acid:[4] In one alternative method, formation of toxic chromyl chloride (CrO2Cl2) fumes during the making of the aforementioned solution were minimized by simply changing the order of addition: a cold solution of pyridine in concentrated hydrochloric acid was added to solid chromium trioxide under stirring.
In particular, it has proven to be highly effective in oxidizing primary and secondary alcohols to aldehydes and ketones, respectively.
The reagent is more selective than the related Jones' Reagent, so there is little chance of over-oxidation to form carboxylic acids if acidified potassium permanganate is used as long as water is not present in the reaction mixture.