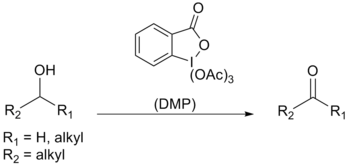
Dess–Martin periodinane
[1][2] This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions (room temperature, neutral pH), shorter reaction times, higher yields, simplified workups, high chemoselectivity, tolerance of sensitive functional groups, and a long shelf life.
One of the reasons for its effectiveness is its high selectivity towards complexation of the hydroxyl group, which allows alcohols to rapidly perform ligand exchange; the first step in the oxidation reaction.
It is believed that the rate of dissociation of the final acetate ligand from the iodine is increased, because of the electron-donating ability of the hydroxyl group (thus weakening the I-OAc bond).
[4] Using the standard Dess–Martin periodinane conditions, alcohols can be oxidized to aldehydes/ketones without affecting furan rings, sulfides, vinyl ethers, and secondary amides.
This moiety has been found in several natural products and due to its high functionality, it could be a valuable synthetic building block in organic synthesis.
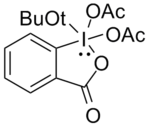
Using the compound shown below produced the desired carbonyls in high yields as the addition of the tert-butoxy group, due to its steric bulk, minimizes these side reactions.