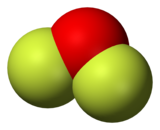
Oxygen difluoride
Oxygen difluoride was first reported in 1929; it was obtained by the electrolysis of molten potassium fluoride and hydrofluoric acid containing small quantities of water.
[7][8] The modern preparation entails the reaction of fluorine with a dilute aqueous solution of sodium hydroxide, with sodium fluoride as a side-product: It is a covalently bonded molecule with a bent molecular geometry and a F-O-F bond angle of 103 degrees.
Oxygen difluoride reacts with water to form hydrofluoric acid: It can oxidize sulphur dioxide to sulfur trioxide and elemental fluorine: However, in the presence of UV radiation, the products are sulfuryl fluoride (SO2F2) and pyrosulfuryl fluoride (S2O5F2): Oxygen difluoride is considered an unsafe gas due to its oxidizing properties.
Other acute poisoning effects include: pulmonary edema, bleeding lungs, headaches, etc.
While OF2 would be a solid at 30 K, the fictional alien lifeforms were described as endothermic, maintaining elevated body temperatures and liquid OF2 blood by radiothermal heating.