Oxygen rebound mechanism
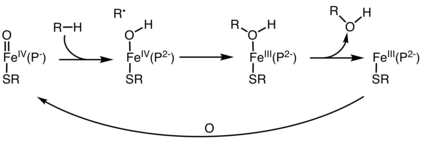
In biochemistry, the oxygen rebound mechanism is the pathway for hydroxylation of organic compounds by iron-containing oxygenases.
Many enzymes effect the hydroxylation of hydrocarbons as a means for biosynthesis, detoxification, gene regulation, and other functions.
These enzymes often utilize Fe-O centers that convert C-H bonds into C-OH groups.
The oxygen rebound mechanism starts with abstraction of H from the hydrocarbon, giving an organic radical and an iron hydroxide.
In the rebound step, the organic radical attacks the Fe-OH center to give an alcohol group, which is bound to Fe as a ligand.