CDKN1B
In addition to this structural similarity the "Cip/Kip" proteins share the functional characteristic of being able to bind several different classes of Cyclin and Cdk molecules.
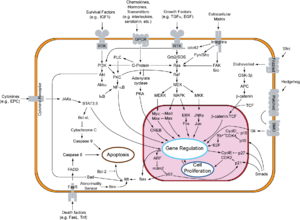
In doing so p27Kip1 inhibits the catalytic activity of Cdk4, which means that it prevents Cdk4 from adding phosphate residues to its principal substrate, the retinoblastoma (pRb) protein.
[6] In general, extracellular growth factors which promote cell division reduce transcription and translation of p27Kip1.
[14] A structured cis-regulatory element has been found in the 5' UTR of the P27 mRNA where it is thought to regulate translation relative to cell cycle progression.
Transcription of the CDKN1B gene is activated by Forkhead box class O family (FoxO) proteins which also acts downstream to promote p27 nuclear localization and decrease levels of COP9 subunit 5(COPS5) which helps in the degradation of p27.
[17] Transcription for p27 is activated by FoxO in response to cytokines, promyelocytic leukaemia proteins, and nuclear Akt signaling.
[17] P27 transcription has also been linked to another tumor suppressor gene, MEN1, in pancreatic islet cells where it promotes CDKN1B expression.
During early G1 proteolysis of p27 is regulated by KIP1 Ubiquitylation Promoting Complex (KPC) which binds to its CDK inhibitory domain.
This occurs when p27 is phosphorylated on Ser(10) which allows for CRM1, a nuclear export carrier protein, to bind to and remove p27 from the nucleus.
[17] These act to accelerate the proteolysis of the p27 protein and allow the cancer cell to undergo rapid division and uncontrolled proliferation.
[17] Many cancer cells also upregulate Skp2 which is known to play an active role in the proteolysis of p27[18] As a result, Skp2 is inversely related to p27 levels and directly correlates with tumor grade in many malignancies.
[22] In breast cancer cytoplasmic p27 reduced RHOA activity which increased a cell's propensity for motility.
[28] Because inhibition of RhoA results in a decrease in both stress fibers and focal adhesion, cell motility is increased.
[32] Mutations in the CDKN1B gene has been reported in families affected by the development of primary hyperparathyroidism and pituitary adenomas, and has been classified MEN4 (multiple endocrine neoplasia, type 4).
[17] Or in contrast, if p27 levels are found to be high in a patient's cancer, their risk for metastasis is higher and the physician can make an informed decision about their treatment plan.
For example, patients with non-small cell lung cancer who were treated with platinum based chemotherapy showed reduced survival if they had low levels of p27.
[39] Similarly low levels of p27 correlated with poor results from adjuvant chemotherapy in breast cancer patients.
[41] This is true for a wide spectrum of cancers including colon, breast, prostate, lung, liver, stomach, and bladder.
[41] Because of the role miRNAs play in p27 regulation, research is underway to determine if antagomiRs that block the activity of the miR221&222 and allow for p27 cell grow inhibition to take place could act as therapeutic cancer drugs.
[42][43] Because hair cell death in the human cochlea is a major cause of hearing loss, the CDKN1B protein could be an important factor in the clinical treatment of deafness.