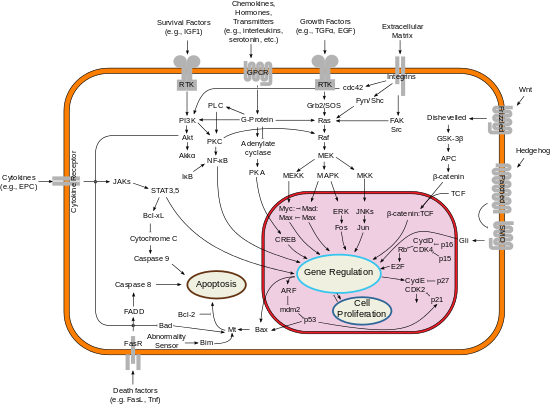
Bcl-2
[10] BCL-2 is localized to the outer membrane of mitochondria, where it plays an important role in promoting cellular survival and inhibiting the actions of pro-apoptotic proteins.
The pro-apoptotic proteins in the BCL-2 family, including Bax and Bak, normally act on the mitochondrial membrane to promote permeabilization and release of cytochrome c and ROS, that are important signals in the apoptosis cascade.
But simultaneous over-expression of Bcl-2 and the proto-oncogene myc may produce aggressive B-cell malignancies including lymphoma.
[18] Other studies have shown that dendritic cell lifespan may be partly controlled by a timer dependent on anti-apoptotic Bcl-2.
In some cases, the presence or absence of Bcl-2 staining in biopsies may be significant for the patient's prognosis or likelihood of relapse.
An antisense drug is a short sequence of modified DNA that hybridises with and inactivates mRNA, preventing the protein from being formed.
[citation needed] Human lymphoma cell proliferation (with t(14;18) translocation) could be inhibited by antisense oligonucleotide targeted at the start codon region of Bcl-2 mRNA.
This compound is part of a group of BH3 mimetic small molecule inhibitors (SMI) that target these Bcl-2 family proteins, but not A1 or Mcl-1.
[24] In animal models, it improves survival, causes tumor regression and cures a high percentage of mice.
[25] In preclinical studies utilizing patient xenografts, ABT-737 showed efficacy for treating lymphoma and other blood cancers.
[27] While clinical responses with navitoclax were promising, mechanistic dose-limiting thrombocytopenia was observed in patients under treatment due to Bcl-xL inhibition in platelets.
[28][29][30] Due to dose-limiting thrombocytopenia of navitoclax as a result of Bcl-xL inhibition, Abbvie successfully developed the highly selective inhibitor venetoclax (ABT-199), which inhibits Bcl-2, but not Bcl-xL or Bcl-w.[31] Clinical trials studied the effects of venetoclax, a BH3-mimetic drug designed to block the function of the Bcl-2 protein, on patients with chronic lymphocytic leukemia (CLL).
[36] In June 2018, the FDA broadened the approval for anyone with CLL or small lymphocytic lymphoma, with or without 17p deletion, still as a second-line treatment.