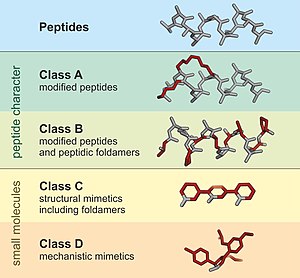
Peptidomimetic
Irrespective of the approach, the altered chemical structure is designed to advantageously adjust the molecular properties such as stability or biological activity.
[1] Introduced modifications usually aim to increase the stability of the peptide, its affinity for a desired binding partner, oral availability or cell permeability.
Rapid degradation, poor oral availability, difficult transportation through cell membranes, nonselective receptor binding, and challenging multistep preparation are the major limitations of peptides as active pharmaceutical ingredients.
[3] Therefore, small protein-like chains called peptidomimetics could be designed and used to mimic native analogs and conceivably exhibit better pharmacological properties.
[3] Many peptidomimetics are utilized as FDA-approved drugs, such as Romidepsin (Istodax), Atazanavir (Reyataz), Saquinavir (Invirase), Oktreotid (Sandostatin), Lanreotide (Somatuline), Plecanatide (Trulance), Ximelagatran (Exanta), Etelcalcetide (Parsabiv), and Bortezomib (Velcade).
[7] Also in 2004, Harran and co-workers reported a dimeric small molecule that mimics the proapoptotic protein Smac (see mitochondrial regulation in apoptosis).
[10] Smac mimetics of this type can sensitize an array of non-small-cell lung cancer cells to conventional chemotherapeutics (e.g. Gemcitabine, Vinorelbine) both in vitro and in mouse xenograft models.