Beta-peptide
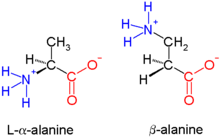
The parent β-amino acid is β-alanine (H2NCH2CH2CO2H), a common natural substance, but most examples feature substituents in place of one or more C-H bonds.
The alkyl substituents at both the α and β positions in a β-amino acid favor a gauche conformation about the bond between the α-carbon and β-carbon.
These conformation types are distinguished by the number of atoms in the hydrogen-bonded ring that is formed in solution; 8-helix, 10-helix, 12-helix, 14-helix,[6] and 10/12-helix have been reported.
[8] β-Peptides have been used to mimic natural peptide-based antibiotics such as magainins, which are highly potent but difficult to use as drugs because they are degraded by proteolytic enzymes.
Named by analogy to the biological α-amino acids, the following have been found naturally: β-alanine, β-leucine, β-lysine, β-arginine, β-glutamate, β-glutamine, β-phenylalanine and β-tyrosine.