Periodate
[3] Or, similarly, from iodides by oxidation with bromine and sodium hydroxide: Modern industrial scale production involves the electrochemical oxidation of iodates, on a lead dioxide (PbO2) anode, with the following standard electrode potential: Metaperiodates are typically prepared by the dehydration of sodium hydrogen periodate with nitric acid,[2] or by dehydrating orthoperiodic acid by heating it to 100 °C under vacuum.
They can also be generated directly from iodates by treatment with other strong oxidizing agents such as hypochlorites: Periodate can exist in a variety of forms in aqueous media, with pH being a controlling factor.
For this reason orthoperiodate is sometimes referred to as the dihydrate of metaperiodate,[citation needed] written IO−4·2H2O; however, this description is not strictly accurate as X-ray crystallography of H5IO6 shows 5 equivalent I−OH groups.
[9] Exact structures vary depending on counter ions, however on average orthoperiodates adopt a slightly deformed octahedral geometry with X-ray diffraction showing I–O bond lengths of 1.89 Å.
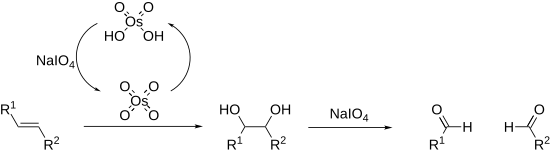
As periodate salts are only readily soluble in water reactions are generally performed in aqueous media.
In extreme cases the periodate may be exchanged for lead tetraacetate which reacts in a similar manner and is soluble in organic solvents (Criegee oxidation).
Periodate cleavage is often utilized in molecular biochemistry for the purposes of modifying saccharide rings, as many five- and six-membered sugars have vicinal diols.
[23][24] Periodate cleavage may be performed on an industrial scale to form dialdehyde starch which has uses in paper production.
[31] In 2013 the US Army announced that it would replace the environmentally harmful chemicals barium nitrate and potassium perchlorate with sodium metaperiodate for use in their tracer ammunition.