Periodic acid
[3] Modern industrial scale production involves the oxidation of a solution of sodium iodate under alkaline conditions, either electrochemically on a PbO2 anode, or by treatment with chlorine:[4] A standard laboratory preparation involves treating a mixture of barium periodate with nitric acid.
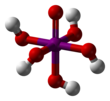
Orthoperiodic acid forms monoclinic crystals (space group P21/n) consisting of a slightly deformed IO6 octahedron interlinked via bridging hydrogens.
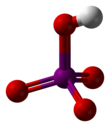
[9][10] The structure of metaperiodic acid also includes IO6 octahedra, however these are connected via cis-edge-sharing with bridging oxygens to form one-dimensional infinite chains.
Most notably periodic acid will cleave vicinal diols into two aldehyde or ketone fragments (Malaprade reaction).
[13] Periodic acid is part of a series of oxyacids in which iodine can assume oxidation states of −1, +1, +3, +5, or +7.