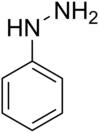
Phenylhydrazine
[5] Phenylhydrazine forms monoclinic prisms that melt to an oil around room temperature which may turn yellow to dark red upon exposure to air.
[1] Phenylhydrazine is miscible with ethanol, diethyl ether, chloroform and benzene.
[6] Phenylhydrazine was the first hydrazine derivative characterized, reported by Hermann Emil Fischer in 1875.
[9] This molecule is also used to induce acute hemolytic anemia in animal models.
Exposure to phenylhydrazine may cause contact dermatitis, hemolytic anemia, and liver damage.