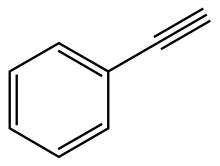
Phenylacetylene
In the laboratory, phenylacetylene can be prepared by elimination of hydrogen bromide from styrene dibromide using sodium amide in ammonia:[3] It can also be prepared by the elimination of hydrogen bromide from bromostyrene using molten potassium hydroxide.
[4] Yet another method involves the Sonogashira coupling of iodobenzene with trimethylsilylacetylene, followed by removal of the trimethylsilyl group using TBAF.
[5] Phenylacetylene is a prototypical terminal acetylene, undergoing many reactions expected of that functional group.
In the presence of base and copper(II) salts, it undergoes oxidative coupling to give diphenylbutadiyne.
[7][8] In the presence of gold or mercury reagents, phenylacetylene hydrates to give acetophenone: