Phosphirenium ion
Phosphirenium ions (R1R2C2PY1Y+2) are a series of organophosphorus compounds containing unsaturated three-membered ring phosphorus (V) heterocycles and σ*-aromaticity is believed to be present in such molecules.
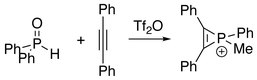
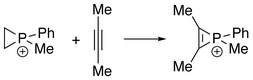
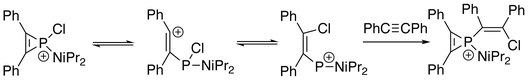
The first series of phosphirenium ions were synthesized by reacting alkynes with methyl- or phenylphosphonous dichloride and aluminum trichloride.
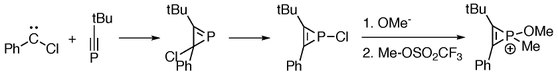
[1] [2+1]-cycloaddition reactions between phosphaalkynes and chlorocarbene give phosphirenes, which serve as starting materials for the generation of phosphirenium species.
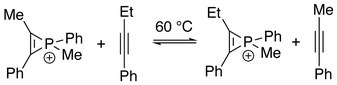
[10] Electronegativity of each substituent on phosphorus plays a role as more electron-donating ones give greater degrees of antiaromatic sigma destabilization.
This has been confirmed by Natural Population Analysis (NPA), where the energy changes of the reactions below were calculated with interactions between the C–C double bond and phosphorus both turned on and off by manipulating Fock matrix elements: Destabilization energies were the differences between corresponding reactions:[10] This series is in accordance with the trend of electronegativity of the ligand atoms.



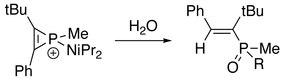
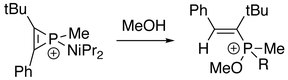
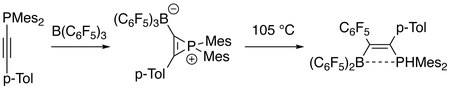
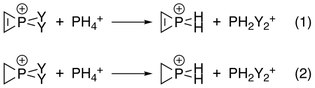

2 C
2 PF +
2 from NBO analysis.