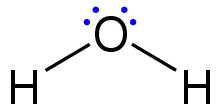
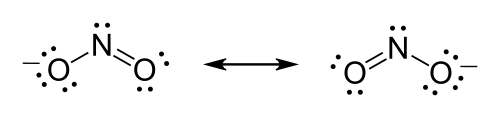
Lewis structure
[4] Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond.
Another simple and general procedure to write Lewis structures and resonance forms has been proposed.
It has uses in determining possible electron re-configuration when referring to reaction mechanisms, and often results in the same sign as the partial charge of the atom, with exceptions.
Each of the different possibilities is superimposed on the others, and the molecule is considered to have a Lewis structure equivalent to some combination of these states.
Single bonds can also be moved in the same way to create resonance structures for hypervalent molecules such as sulfur hexafluoride, which is the correct description according to quantum chemical calculations instead of the common expanded octet model.
In condensed structural formulas, many or even all of the covalent bonds may be left out, with subscripts indicating the number of identical groups attached to a particular atom.
Despite their simplicity and development in the early twentieth century, when understanding of chemical bonding was still rudimentary, Lewis structures capture many of the key features of the electronic structure of a range of molecular systems, including those of relevance to chemical reactivity.
This is especially true in the field of organic chemistry, where the traditional valence-bond model of bonding still dominates, and mechanisms are often understood in terms of curve-arrow notation superimposed upon skeletal formulae, which are shorthand versions of Lewis structures.
Due to the greater variety of bonding schemes encountered in inorganic and organometallic chemistry, many of the molecules encountered require the use of fully delocalized molecular orbitals to adequately describe their bonding, making Lewis structures comparatively less important (although they are still common).
There are simple and archetypal molecular systems for which a Lewis description, at least in unmodified form, is misleading or inaccurate.