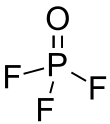
Phosphoryl fluoride
Phosphoryl fluoride (commonly called phosphorus oxyfluoride) is a compound with the chemical formula POF3.
It is a colorless gas that hydrolyzes rapidly.
Phosphorus oxyfluoride is the progenitor of the simple fluorophosphoric acids by hydrolysis.
The sequence starts with difluorophosphoric acid: The next steps give monofluorophosphoric acid and phosphoric acid: Phosphoryl fluoride combines with dimethylamine to produce dimethylaminophosphoryl difluoride (H3C−)2N−P(=O)F2 and difluorophosphate and hexafluorophosphate ions.
[2] This inorganic compound–related article is a stub.